Prognostically relevant gene signatures of high-grade serous ovarian carcinoma
- PMID: 23257362
- PMCID: PMC3533304
- DOI: 10.1172/JCI65833
Prognostically relevant gene signatures of high-grade serous ovarian carcinoma
Abstract
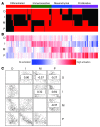
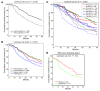
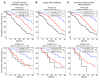
Because of the high risk of recurrence in high-grade serous ovarian carcinoma (HGS-OvCa), the development of outcome predictors could be valuable for patient stratification. Using the catalog of The Cancer Genome Atlas (TCGA), we developed subtype and survival gene expression signatures, which, when combined, provide a prognostic model of HGS-OvCa classification, named "Classification of Ovarian Cancer" (CLOVAR). We validated CLOVAR on an independent dataset consisting of 879 HGS-OvCa expression profiles. The worst outcome group, accounting for 23% of all cases, was associated with a median survival of 23 months and a platinum resistance rate of 63%, versus a median survival of 46 months and platinum resistance rate of 23% in other cases. Associating the outcome prediction model with BRCA1/BRCA2 mutation status, residual disease after surgery, and disease stage further optimized outcome classification. Ovarian cancer is a disease in urgent need of more effective therapies. The spectrum of outcomes observed here and their association with CLOVAR signatures suggests variations in underlying tumor biology. Prospective validation of the CLOVAR model in the context of additional prognostic variables may provide a rationale for optimal combination of patient and treatment regimens.
Figures
Comment in
-
Gynaecological cancer: CLOVAR validates prognostic gene-expression signature.Nat Rev Clin Oncol. 2013 Feb;10(2):68. doi: 10.1038/nrclinonc.2013.2. Epub 2013 Jan 22. Nat Rev Clin Oncol. 2013. PMID: 23337919 No abstract available.
References
-
- Markman M, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21(13):2460–2465. doi: 10.1200/JCO.2003.07.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

