Sputum hyaluronan and versican in severe eosinophilic asthma
- PMID: 23257685
- PMCID: PMC4059000
- DOI: 10.1159/000343031
Sputum hyaluronan and versican in severe eosinophilic asthma
Abstract
Background: We examined levels of hyaluronan, a matrix glycosaminoglycan and versican, a matrix proteoglycan, in the sputum of asthmatics treated with mepolizumab (anti-IL-5 monoclonal antibody) versus placebo to evaluate the utility of these measurements as possible biomarkers of asthma control and airway remodeling.
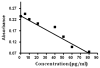
Methods: Patients with severe, prednisone-dependent asthma received either mepolizumab or placebo as described in a previously published randomized, double-blind, placebo-controlled study. We measured hyaluronan and versican levels by enzyme-linked immunosorbent assay in sputum collected before and after the 16-week treatment phase. Patients underwent a predefined prednisone tapering schedule if they remained exacerbation free, and sputum eosinophil percentage, asthma control questionnaire (ACQ) and spirometry were monitored.
Results: After 6 months of mepolizumab therapy and prednisone tapering, there was a significant increase in sputum hyaluronan in the placebo group compared with baseline (p = 0.003). In contrast, there was a significant decrease in sputum hyaluronan in the active treatment group compared with placebo (p = 0.007), which correlated with improvements in percent forced expiratory volume in 1 s (FEV1%) (p = 0.001) and ACQ scores (p = 0.009) as well as a decrease in sputum eosinophils (p = 0.02). There was a nonsignificant increase in sputum versican in the placebo group (p = 0.16), a decrease in the mepolizumab group (p = 0.13) and a significant inverse correlation between versican reduction and FEV1% improvement (p = 0.03).
Conclusions: Sputum hyaluronan values are reduced with mepolizumab therapy and correlate with improved clinical and spirometry values, suggesting this measurement may serve as a noninvasive biomarker of asthma control.
Copyright © 2012 S. Karger AG, Basel.
Figures

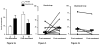
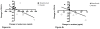
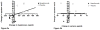
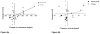
References
-
- Johnson PR, Burgess JK. Airway smooth muscle and fibroblasts in the pathogenesis of asthma. Curr Allergy Asthma Rep. 2004;4(2):102–8. - PubMed
-
- Parameswaran K, Willems-Widyastuti A, Alagappan VK, Radford K, Kranenburg AR, Sharma HS. Role of extracellular matrix and its regulators in human airway smooth muscle biology. Cell Biochem Biophys. 2006;44(1):139–46. - PubMed
-
- Yamauchi K, Inoue H. Airway remodeling in asthma and irreversible airflow limitation-ECM deposition in airway and possible therapy for remodeling- Allergol Int. 2007;56(4):321–9. - PubMed
-
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, function and turnover. J Intern Med. 1997;242(10):27–33. - PubMed
-
- Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. Biol Chem. 2002;277(7):4585–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

