Chemical probing of glycans in cells and organisms
- PMID: 23257905
- PMCID: PMC3641795
- DOI: 10.1039/c2cs35416k
Chemical probing of glycans in cells and organisms
Abstract
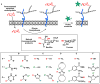
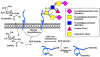
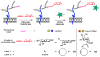
Among the four major building blocks of life, glycans play essential roles in numerous physiological and pathological processes. Due to their non-templated biosynthesis, advances towards elucidating the molecular details of glycan functions are relatively slow compared with the pace of protein and nucleic acid research. Over the past 30 years, chemical tools have emerged as powerful allies to genetics and molecular biology in the study of glycans in their native environment. This tutorial review will provide an overview of the recent technological developments in the field, as well as the progress in the application of these techniques to probe glycans in cells and organisms.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

