Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions
- PMID: 23257984
- PMCID: PMC3612968
- DOI: 10.1038/nprot.2012.150
Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions
Abstract
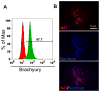
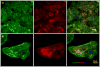
The protocol described here efficiently directs human pluripotent stem cells (hPSCs) to functional cardiomyocytes in a completely defined, growth factor- and serum-free system by temporal modulation of regulators of canonical Wnt signaling. Appropriate temporal application of a glycogen synthase kinase 3 (GSK3) inhibitor combined with the expression of β-catenin shRNA or a chemical Wnt inhibitor is sufficient to produce a high yield (0.8-1.3 million cardiomyocytes per cm(2)) of virtually pure (80-98%) functional cardiomyocytes in 14 d from multiple hPSC lines without cell sorting or selection. Qualitative (immunostaining) and quantitative (flow cytometry) characterization of differentiated cells is described to assess the expression of cardiac transcription factors and myofilament proteins. Flow cytometry of BrdU incorporation or Ki67 expression in conjunction with cardiac sarcomere myosin protein expression can be used to determine the proliferative capacity of hPSC-derived cardiomyocytes. Functional human cardiomyocytes differentiated via these protocols may constitute a potential cell source for heart disease modeling, drug screening and cell-based therapeutic applications.
Conflict of interest statement
T.J.K. is a founder and consultant for Cellular Dynamics International, a company that uses human stem cells for drug testing. All the other authors declare no competing financial interests.
Figures


References
-
- Graichen R, et al. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–370. - PubMed
-
- Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. - PubMed
-
- Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

