Neonatal hyperoxia increases sensitivity of adult mice to bleomycin-induced lung fibrosis
- PMID: 23258231
- PMCID: PMC3604066
- DOI: 10.1165/rcmb.2012-0238OC
Neonatal hyperoxia increases sensitivity of adult mice to bleomycin-induced lung fibrosis
Abstract
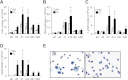
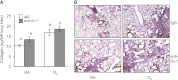
Supplemental oxygen used to treat infants born prematurely constitutes a major risk factor for long-term deficits in lung function and host defense against respiratory infections. Likewise, neonatal oxygen exposure results in alveolar simplification in adult mice, and enhances leukocyte recruitment and fibrosis when adult mice are infected with a sublethal dose of influenza A virus. Because pulmonary fibrosis was not observed in infected adult mice exposed to room air as neonates, previous neonatal oxygen exposure may have reprogrammed how the adult lung responds to epithelial injury. By administering bleomycin to adult mice exposed to room air or hyperoxia as neonates, we tested the hypothesis that neonatal hyperoxia enhances fibrosis when the epithelium is injured by direct fibrotic stimulus. Increased sensitivity to bleomycin-induced lung fibrosis was observed in adult mice exposed to neonatal hyperoxia, and was associated with increased numbers of leukocytes and an accumulation of active transforming growth factor (TGF)-β1 in the lung. Fate mapping of the respiratory epithelium revealed that the epithelial-mesenchymal transition was not a significant source of fibroblasts in room air-exposed or oxygen-exposed mice treated with bleomycin. Instead, the treatment of mice with anti-Gr-1 antibody that depletes neutrophils and myeloid-derived suppressor cells reduced the early activation of TGF-β1 and attenuated hyperoxia-enhanced fibrosis. Because bleomycin and influenza A virus both cause epithelial injury, understanding how neonatal hyperoxia reprograms the epithelial response to these two different injurious agents could lead to new therapeutic opportunities for treating lung diseases attributed to prematurity.
Figures





References
-
- Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol 2006;30:179–184 - PubMed
-
- Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, Richardson DK. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr 2004;144:799–803 - PubMed
-
- Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics 2006;118:108–113 - PubMed
-
- Robin B, Kim YJ, Huth J, Klocksieben J, Torres M, Tepper RS, Castile RG, Solway J, Hershenson MB, Goldstein-Filbrun A. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol 2004;37:236–242 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES-017250/ES/NIEHS NIH HHS/United States
- HL-091968/HL/NHLBI NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- ES-01247/ES/NIEHS NIH HHS/United States
- HL-097141/HL/NHLBI NIH HHS/United States
- R01 ES007026/ES/NIEHS NIH HHS/United States
- R01 HL091968/HL/NHLBI NIH HHS/United States
- ES-07026/ES/NIEHS NIH HHS/United States
- T32 HL066988/HL/NHLBI NIH HHS/United States
- R01 ES017250/ES/NIEHS NIH HHS/United States
- R01 HL097141/HL/NHLBI NIH HHS/United States
- HL-66988/HL/NHLBI NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

