Rac1 and rac3 GTPases control synergistically the development of cortical and hippocampal GABAergic interneurons
- PMID: 23258346
- PMCID: PMC3977619
- DOI: 10.1093/cercor/bhs402
Rac1 and rac3 GTPases control synergistically the development of cortical and hippocampal GABAergic interneurons
Abstract
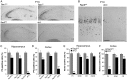
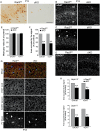
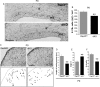
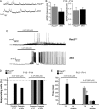
The intracellular mechanisms driving postmitotic development of cortical γ-aminobutyric acid (GABA)ergic interneurons are poorly understood. We have addressed the function of Rac GTPases in cortical and hippocampal interneuron development. Developing neurons express both Rac1 and Rac3. Previous work has shown that Rac1 ablation does not affect the development of migrating cortical interneurons. Analysis of mice with double deletion of Rac1 and Rac3 shows that these GTPases are required during postmitotic interneuron development. The number of parvalbumin-positive cells was affected in the hippocampus and cortex of double knockout mice. Rac depletion also influences the maturation of interneurons that reach their destination, with reduction of inhibitory synapses in both hippocampal CA1 and cortical pyramidal cells. The decreased number of cortical migrating interneurons and their altered morphology indicate a role of Rac1 and Rac3 in regulating the motility of cortical interneurons, thus interfering with their final localization. While electrophysiological passive and active properties of pyramidal neurons including membrane capacity, resting potential, and spike amplitude and duration were normal, these cells showed reduced spontaneous inhibitory currents and increased excitability. Our results show that Rac1 and Rac3 contribute synergistically to postmitotic development of specific populations of GABAergic cells, suggesting that these proteins regulate their migration and differentiation.
Keywords: GABAergic interneurons; Rac GTPases; cortex; hippocampus; neuronal migration.
Figures


Similar articles
-
Loss of Either Rac1 or Rac3 GTPase Differentially Affects the Behavior of Mutant Mice and the Development of Functional GABAergic Networks.Cereb Cortex. 2016 Feb;26(2):873-890. doi: 10.1093/cercor/bhv274. Epub 2015 Nov 17. Cereb Cortex. 2016. PMID: 26582364 Free PMC article.
-
Rac-GTPases Regulate Microtubule Stability and Axon Growth of Cortical GABAergic Interneurons.Cereb Cortex. 2015 Sep;25(9):2370-82. doi: 10.1093/cercor/bhu037. Epub 2014 Mar 13. Cereb Cortex. 2015. PMID: 24626607 Free PMC article.
-
Rac1 and Rac3 GTPases and TPC2 are required for axonal outgrowth and migration of cortical interneurons.J Cell Sci. 2023 Mar 15;136(6):jcs260373. doi: 10.1242/jcs.260373. Epub 2023 Mar 10. J Cell Sci. 2023. PMID: 36744839
-
Roles of Rac1 and Rac3 GTPases during the development of cortical and hippocampal GABAergic interneurons.Front Cell Neurosci. 2014 Sep 25;8:307. doi: 10.3389/fncel.2014.00307. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25309333 Free PMC article. Review.
-
The Rac3 GTPase in Neuronal Development, Neurodevelopmental Disorders, and Cancer.Cells. 2019 Sep 11;8(9):1063. doi: 10.3390/cells8091063. Cells. 2019. PMID: 31514269 Free PMC article. Review.
Cited by
-
Epilepsy and autophagy modulators: a therapeutic split.Autophagy. 2025 Sep;21(9):1863-1887. doi: 10.1080/15548627.2025.2506292. Epub 2025 May 30. Autophagy. 2025. PMID: 40375490 Free PMC article. Review.
-
RAC3 Promotes Proliferation, Migration and Invasion via PYCR1/JAK/STAT Signaling in Bladder Cancer.Front Mol Biosci. 2020 Aug 31;7:218. doi: 10.3389/fmolb.2020.00218. eCollection 2020. Front Mol Biosci. 2020. PMID: 33062641 Free PMC article.
-
Tet3 regulates cellular identity and DNA methylation in neural progenitor cells.Cell Mol Life Sci. 2020 Jul;77(14):2871-2883. doi: 10.1007/s00018-019-03335-7. Epub 2019 Oct 23. Cell Mol Life Sci. 2020. PMID: 31646359 Free PMC article.
-
Rho GTPase isoforms in cell motility: Don't fret, we have FRET.Cell Adh Migr. 2014;8(6):526-34. doi: 10.4161/cam.29712. Cell Adh Migr. 2014. PMID: 25482645 Free PMC article. Review.
-
Erythropoietin Stimulates GABAergic Maturation in the Mouse Hippocampus.eNeuro. 2021 Feb 11;8(1):ENEURO.0006-21.2021. doi: 10.1523/ENEURO.0006-21.2021. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33495244 Free PMC article.
References
-
- Alcantara S, de Lecea L, Del Rio JA, Ferrer I, Soriano E. Transient colocalization of parvalbumin and calbindin D28k in the postnatal cerebral cortex: evidence for a phenotypic shift in developing nonpyramidal neurons. Eur J Neurosci. 1996;8:1329–1339. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

