Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets
- PMID: 23258846
- PMCID: PMC3548586
- DOI: 10.1093/neuonc/nos298
Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets
Abstract
Background: Gliomas exhibit high glycolytic rates, and monocarboxylate transporters (MCTs) play a major role in the maintenance of the glycolytic metabolism through the proton-linked transmembrane transport of lactate. However, their role in gliomas is poorly studied. Thus, we aimed to characterize the expression of MCT1, MCT4, and their chaperone CD147 and to assess the therapeutic impact of MCT inhibition in gliomas.
Methods: MCTs and CD147 expressions were characterized by immunohistochemistry in nonneoplastic brain and glioma samples. The effect of CHC (MCT inhibitor) and MCT1 silencing was assessed in in vitro and in vivo glioblastoma models.
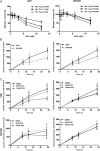
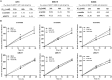
Results: MCT1, MCT4, and CD147 were overexpressed in the plasma membrane of glioblastomas, compared with diffuse astrocytomas and nonneoplastic brain. CHC decreased glycolytic metabolism, migration, and invasion and induced cell death in U251 cells (more glycolytic) but only affected proliferation in SW1088 (more oxidative). The effectiveness of CHC in glioma cells appears to be dependent on MCT membrane expression. MCT1 downregulation showed similar effects on different glioma cells, supporting CHC as an MCT1 inhibitor. There was a synergistic effect when combining CHC with temozolomide treatment in U251 cells. In the CAM in vivo model, CHC decreased the size of tumors and the number of blood vessels formed.
Conclusions: This is the most comprehensive study reporting the expression of MCTs and CD147 in gliomas. The MCT1 inhibitor CHC exhibited anti-tumoral and anti-angiogenic activity in gliomas and, of importance, enhanced the effect of temozolomide. Thus, our results suggest that development of therapeutic approaches targeting MCT1 may be a promising strategy in glioblastoma treatment.
Figures






References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24(2):68–72. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

