The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole
- PMID: 23259056
- PMCID: PMC3522883
- DOI: 10.1242/bio.20122840
The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole
Abstract
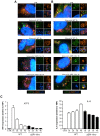
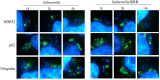
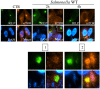
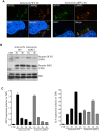
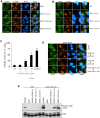
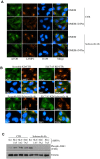
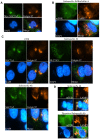
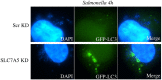
Bacterial invasion results in the rapid induction of an acute state of cytosolic amino acid (AA) starvation, provoked by host membrane damage. Bacteria-induced AA starvation, in turn, down-regulates mTOR signaling while triggering autophagy and the integrated stress response pathway dependent on GCN2, eIF2α and ATF3. In Salmonella-infected cells, we now demonstrate that the host AA starvation response program depended on the Salmonella pathogenicity island (SPI)-1, the activity of which was required to damage the Salmonella-containing vacuole (SCV) in the early stage of infection. At a later stage (3-4 hour post-infection), the progressive recruitment of mTOR to the surface of the SCV appeared to be independent of the activity of SPI-2 and of SCV positioning in the cell. Instead, mTOR localization to the SCV required the activity of host AA transporters SLC1A5, SLC3A2 and SLC7A5, resulting in bacterial escape from autophagy. These results expand our understanding of the mechanisms underlying the AA starvation response in Salmonella-infected cells.
Keywords: Amino acid starvation; Salmonella; mTOR.
Conflict of interest statement
Figures
Similar articles
-
Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program.Cell Host Microbe. 2012 Jun 14;11(6):563-75. doi: 10.1016/j.chom.2012.04.012. Cell Host Microbe. 2012. PMID: 22704617
-
Bacterial autophagy: the trigger, the target and the timing.Autophagy. 2012 Dec;8(12):1848-50. doi: 10.4161/auto.21863. Epub 2012 Aug 29. Autophagy. 2012. PMID: 22932645 Free PMC article.
-
Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole.J Biol Chem. 2006 Apr 21;281(16):11374-83. doi: 10.1074/jbc.M509157200. Epub 2006 Feb 22. J Biol Chem. 2006. PMID: 16495224
-
Salmonella-containing vacuoles: directing traffic and nesting to grow.Traffic. 2008 Dec;9(12):2022-31. doi: 10.1111/j.1600-0854.2008.00827.x. Epub 2008 Oct 8. Traffic. 2008. PMID: 18778407 Review.
-
Salmonella modulation of the phagosome membrane, role of SseJ.Cell Microbiol. 2015 Mar;17(3):333-41. doi: 10.1111/cmi.12420. Epub 2015 Feb 23. Cell Microbiol. 2015. PMID: 25620407 Review.
Cited by
-
Autophagy-Related Proteins Target Ubiquitin-Free Mycobacterial Compartment to Promote Killing in Macrophages.Front Cell Infect Microbiol. 2016 May 11;6:53. doi: 10.3389/fcimb.2016.00053. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27242971 Free PMC article.
-
Rubicon-Dependent Lc3 Recruitment to Salmonella-Containing Phagosomes Is a Host Defense Mechanism Triggered Independently From Major Bacterial Virulence Factors.Front Cell Infect Microbiol. 2019 Aug 2;9:279. doi: 10.3389/fcimb.2019.00279. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31428591 Free PMC article.
-
Akt Inhibition Promotes Autophagy and Clearance of Group B Streptococcus from the Alveolar Epithelium.Pathogens. 2022 Sep 30;11(10):1134. doi: 10.3390/pathogens11101134. Pathogens. 2022. PMID: 36297190 Free PMC article.
-
Pathogens MenTORing Macrophages and Dendritic Cells: Manipulation of mTOR and Cellular Metabolism to Promote Immune Escape.Cells. 2020 Jan 9;9(1):161. doi: 10.3390/cells9010161. Cells. 2020. PMID: 31936570 Free PMC article. Review.
-
Lactobacillus rhamnosus GG Affects Microbiota and Suppresses Autophagy in the Intestines of Pigs Challenged with Salmonella Infantis.Front Microbiol. 2018 Jan 17;8:2705. doi: 10.3389/fmicb.2017.02705. eCollection 2017. Front Microbiol. 2018. PMID: 29403451 Free PMC article.
References
-
- Birmingham C. L., Brumell J. H. (2006). Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy 2, 156–158. - PubMed
-
- Birmingham C. L., Canadien V., Gouin E., Troy E. B., Yoshimori T., Cossart P., Higgins D. E., Brumell J. H. (2007). Listeria monocytogenes evades killing by autophagy during colonization of host cells. Autophagy 3, 442–451. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

