Iron-sulfur cluster binding by mitochondrial monothiol glutaredoxin-1 of Trypanosoma brucei: molecular basis of iron-sulfur cluster coordination and relevance for parasite infectivity
- PMID: 23259530
- PMCID: PMC3739951
- DOI: 10.1089/ars.2012.4859
Iron-sulfur cluster binding by mitochondrial monothiol glutaredoxin-1 of Trypanosoma brucei: molecular basis of iron-sulfur cluster coordination and relevance for parasite infectivity
Abstract
Aims: Monothiol glutaredoxins (1-C-Grxs) are small proteins linked to the cellular iron and redox metabolism. Trypanosoma brucei brucei, model organism for human African trypanosomiasis, expresses three 1-C-Grxs. 1-C-Grx1 is a highly abundant mitochondrial protein capable to bind an iron-sulfur cluster (ISC) in vitro using glutathione (GSH) as cofactor. We here report on the functional and structural analysis of 1-C-Grx1 in relation to its ISC-binding properties.
Results: An N-terminal extension unique to 1-C-Grx1 from trypanosomatids affects the oligomeric structure and the ISC-binding capacity of the protein. The active-site Cys104 is essential for ISC binding, and the parasite-specific glutathionylspermidine and trypanothione can replace GSH as the ligands of the ISC. Interestingly, trypanothione forms stable protein-free ISC species that in vitro are incorporated into the dithiol T. brucei 2-C-Grx1, but not 1-C-Grx1. Overexpression of the C104S mutant of 1-C-Grx1 impairs disease progression in a mouse model. The structure of the Grx-domain of 1-C-Grx1 was solved by nuclear magnetic resonance spectroscopy. Despite the fact that several residues--which in other 1-C-Grxs are involved in the noncovalent binding of GSH--are conserved, different physicochemical approaches did not reveal any specific interaction between 1-C-Grx1 and free thiol ligands.
Innovation: Parasite Grxs are able to coordinate an ISC formed with trypanothione, suggesting a new mechanism of ISC binding and a novel function for the parasite-specific dithiol. The first 3D structure and in vivo relevance of a 1-C-Grx from a pathogenic protozoan are reported.
Conclusion: T. brucei 1-C-Grx1 is indispensable for mammalian parasitism and utilizes a new mechanism for ISC binding.
Figures



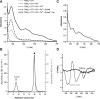




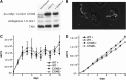

References
-
- Ariyanayagam MR. Fairlamb AH. Ovothiol and trypanothione as antioxidants in trypanosomatids. Mol Biochem Parasitol. 2001;115:189–198. - PubMed
-
- Barbato G. Ikura M. Kay LE. Pastor RW. Bax A. Backbone dynamics of calmodulin studied by 15N relaxation using inverse detected two-dimensional NMR spectroscopy: the central helix is flexible. Biochemistry. 1992;31:5269–5278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

