Bioinformatic identification of Mycobacterium tuberculosis proteins likely to target host cell mitochondria: virulence factors?
- PMID: 23259719
- PMCID: PMC3563495
- DOI: 10.1186/2042-5783-2-9
Bioinformatic identification of Mycobacterium tuberculosis proteins likely to target host cell mitochondria: virulence factors?
Abstract
Background: M. tuberculosis infection either induces or inhibits host cell death, depending on the bacterial strain and the cell microenvironment. There is evidence suggesting a role for mitochondria in these processes.On the other hand, it has been shown that several bacterial proteins are able to target mitochondria, playing a critical role in bacterial pathogenesis and modulation of cell death. However, mycobacteria-derived proteins able to target host cell mitochondria are less studied.
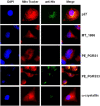
Results: A bioinformaic analysis based on available genomic sequences of the common laboratory virulent reference strain Mycobacterium tuberculosis H37Rv, the avirulent strain H37Ra, the clinical isolate CDC1551, and M. bovis BCG Pasteur strain 1173P2, as well as of suitable bioinformatic tools (MitoProt II, PSORT II, and SignalP) for the in silico search for proteins likely to be secreted by mycobacteria that could target host cell mitochondria, showed that at least 19 M. tuberculosis proteins could possibly target host cell mitochondria. We experimentally tested this bioinformatic prediction on four M. tuberculosis recombinant proteins chosen from this list of 19 proteins (p27, PE_PGRS1, PE_PGRS33, and MT_1866). Confocal microscopy analyses showed that p27, and PE_PGRS33 proteins colocalize with mitochondria.
Conclusions: Based on the bioinformatic analysis of whole M. tuberculosis genome sequences, we propose that at least 19 out of 4,246 M. tuberculosis predicted proteins would be able to target host cell mitochondria and, in turn, control mitochondrial physiology. Interestingly, such a list of 19 proteins includes five members of a mycobacteria specific family of proteins (PE/PE_PGRS) thought to be virulence factors, and p27, a well known virulence factor. P27, and PE_PGRS33 proteins experimentally showed to target mitochondria in J774 cells. Our results suggest a link between mitochondrial targeting of M. tuberculosis proteins and virulence.
Figures

References
-
- WHO. report 2010, http://www.who.int/tb/publications/global_report/2010.
-
- Papatheodorou P, Domanska G, Oxle M, Mathieu J, Selchow O, Kenny B, Rassow J. The enterophatogenic Escherichia coli (EPEC) Map effector is imported into the mitochondrial matrix by the TOM/Hsp70 system and alters organelle morphology. Cell Microbiol. 2006;8:677–689. doi: 10.1111/j.1462-5822.2005.00660.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
