Hepatitis C virus infection of a thyroid cell line: implications for pathogenesis of hepatitis C virus and thyroiditis
- PMID: 23259732
- PMCID: PMC3704108
- DOI: 10.1089/thy.2012.0507
Hepatitis C virus infection of a thyroid cell line: implications for pathogenesis of hepatitis C virus and thyroiditis
Abstract
Background: Autoimmune and non-autoimmune thyroiditis frequently occur in persons with hepatitis C virus (HCV) infection. Treatment with interferon alpha (IFNα) is also associated with significant risk for the development of thyroiditis. To explore HCV-thyroid interactions at a cellular level, we evaluated whether a human thyroid cell line (ML1) could be infected productively with HCV in vitro.
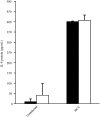
Methods and results: ML1 cells showed robust surface expression of the major HCV receptor CD81. Using a highly sensitive, strand-specific reverse transcription polymerase chain reaction assay, positive-sense and negative-sense HCV RNA were detected in ML1 cell lysates at days 3, 7, and 14 postinfection with HCV. HCV core protein was expressed at high levels in ML1 supernatants at days 1, 3, 5, 7, and 14 postinfection. The nonstructural protein NS5A was also detected in ML1 cell lysates by Western blotting. HCV entry into ML1 cells was shown to be dependent on the HCV entry factors CD81 and SR-B1/CLA1, while IFNα inhibited HCV replication in ML1 cells in a dose-dependent manner. Supernatants from HCV-infected ML1 cells were able to infect fresh ML1 cells productively, suggesting that infectious virions could be transferred from infected to naïve thyroid cells in vivo. Additionally, HCV infection of ML1 cells led to increased expression of the pro-inflammatory cytokine IL-8.
Conclusions: For the first time, we have demonstrated that HCV can infect human thyroid cells in vitro. These findings strongly suggest that HCV infection of thyrocytes may play a role in the association between chronic HCV infection and thyroid autoimmunity. Furthermore, the thyroid may serve as an extrahepatic reservoir for HCV viral replication, thus contributing to the persistence of viral infection and to the development of thyroid autoimmunity.
Figures







References
-
- Blackard JT. Kemmer N. Sherman K. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44:15–22. - PubMed
-
- Cacoub P. Poynard T. Ghillani P. Charlotte F. Olivi M. Piette JC. Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204–2212. - PubMed
-
- Antonelli A. Ferri C. Pampana A. Fallahi P. Nesti C. Pasquini M. Marchi S. Ferrannini E. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117:10–13. - PubMed
-
- Bartolomé J. Rodríguez-Iñigo E. Quadros P. Vidal S. Pascual-Miguelañez I. Rodríguez-Montes JA. García-Sancho L. Carreño V. Detection of hepatitis C virus in thyroid tissue from patients with chronic HCV infection. J Med Virol. 2008;80:1588–1594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

