Invasive pneumococcal disease after routine pneumococcal conjugate vaccination in children, England and Wales
- PMID: 23259937
- PMCID: PMC3557991
- DOI: 10.3201/eid1901.120741
Invasive pneumococcal disease after routine pneumococcal conjugate vaccination in children, England and Wales
Abstract
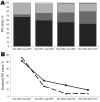
We assessed known risk factors, clinical presentation, and outcome of invasive pneumococcal disease (IPD) in children 3-59 months of age after introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in England and Wales. During September 2006-March 2010, a total of 1,342 IPD episodes occurred in 1,332 children; 14.9% (198/1,332) had comorbidities. Compared with IPD caused by PCV7 serotypes (44/248; 17.7%), comorbidities were less common for the extra 3 serotypes in the 10-valent vaccine (15/299; 5.0%) but similar to the 3 additional PCV13 serotypes (45/336; 13.4%) and increased for the 11 extra serotypes in 23-valent polysaccharide vaccine (PPV23) (39/186; 21.0%) and non-PPV23 serotypes (38/138; 27.5%). Fifty-two (3.9%) cases resulted from PCV7 failure; 9 (0.7%) case-patients had recurrent IPD. Case-fatality rate was 4.4% (58/1,332) but higher for meningitis (11.0%) and children with comorbidities (9.1%). Thus, comorbidities were more prevalent in children with IPD caused by non-PCV13 serotypes and were associated with increased case fatality.
Figures
References
-
- Department of Health. Planned changes to the routine Childhood Immunisation Programme [cited 2012 Aug 21]. http://www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/De...
-
- COVER programme, October to December 2008: quarterly vaccination coverage statistics for children aged up to five years in the United Kingdom. Health Protection Reports 2009;3:8–15 [cited 2012 Aug 21]. http://www.hpa.org.uk/hpr/archives/2009/hpr1209.pdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
