Functional properties of cortical feedback projections to the olfactory bulb
- PMID: 23259952
- PMCID: PMC3530161
- DOI: 10.1016/j.neuron.2012.10.028
Functional properties of cortical feedback projections to the olfactory bulb
Abstract
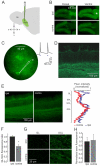
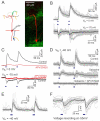
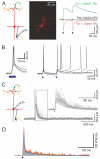
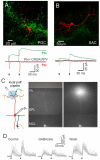
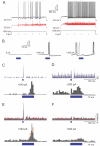
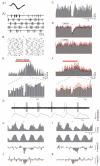
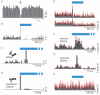
Sensory perception is not a simple feed-forward process, and higher brain areas can actively modulate information processing in "lower" areas. We used optogenetic methods to examine how cortical feedback projections affect circuits in the first olfactory processing stage, the olfactory bulb. Selective activation of back projections from the anterior olfactory nucleus/cortex (AON) revealed functional glutamatergic synaptic connections on several types of bulbar interneurons. Unexpectedly, AON axons also directly depolarized mitral cells (MCs), enough to elicit spikes reliably in a time window of a few milliseconds. MCs received strong disynaptic inhibition, a third of which arises in the glomerular layer. Activating feedback axons in vivo suppressed spontaneous as well as odor-evoked activity of MCs, sometimes preceded by a temporally precise increase in firing probability. Our study indicates that cortical feedback can shape the activity of bulbar output neurons by enabling precisely timed spikes and enforcing broad inhibition to suppress background activity.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Comment in
-
There and back again: the corticobulbar loop.Neuron. 2012 Dec 20;76(6):1045-7. doi: 10.1016/j.neuron.2012.12.006. Neuron. 2012. PMID: 23259940
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

