Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation
- PMID: 23259953
- PMCID: PMC3530172
- DOI: 10.1016/j.neuron.2012.10.036
Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation
Abstract
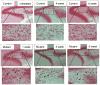
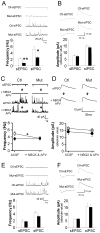
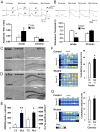
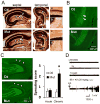
Although excitatory mossy cells of the hippocampal hilar region are known to project both to dentate granule cells and to interneurons, it is as yet unclear whether mossy cell activity's net effect on granule cells is excitatory or inhibitory. To explore their influence on dentate excitability and hippocampal function, we generated a conditional transgenic mouse line, using the Cre/loxP system, in which diphtheria toxin receptor was selectively expressed in mossy cells. One week after injecting toxin into this line, mossy cells throughout the longitudinal axis were degenerated extensively, theta wave power of dentate local field potentials increased during exploration, and deficits occurred in contextual discrimination. By contrast, we detected no epileptiform activity, spontaneous behavioral seizures, or mossy-fiber sprouting 5-6 weeks after mossy cell degeneration. These results indicate that the net effect of mossy cell excitation is to inhibit granule cell activity and enable dentate pattern separation.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. - PubMed
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
-
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. - PubMed
-
- Bilkey DK, Goddard GV. Septohippocampal and commissural pathways antagonistically control inhibitory interneurons in the dentate gyrus. Brain Res. 1987;405:320–325. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

