Detergent properties influence the stability of the glycophorin A transmembrane helix dimer in lysophosphatidylcholine micelles
- PMID: 23260047
- PMCID: PMC3525851
- DOI: 10.1016/j.bpj.2012.11.004
Detergent properties influence the stability of the glycophorin A transmembrane helix dimer in lysophosphatidylcholine micelles
Abstract
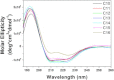
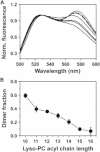
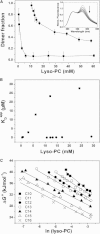
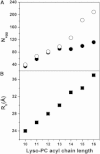
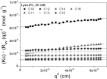
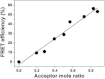
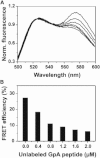
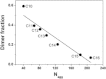
Detergents might affect membrane protein structures by promoting intramolecular interactions that are different from those found in native membrane bilayers, and fine-tuning detergent properties can be crucial for obtaining structural information of intact and functional transmembrane proteins. To systematically investigate the influence of the detergent concentration and acyl-chain length on the stability of a transmembrane protein structure, the stability of the human glycophorin A transmembrane helix dimer has been analyzed in lyso-phosphatidylcholine micelles of different acyl-chain length. While our results indicate that the transmembrane protein is destabilized in detergents with increasing chain-length, the diameter of the hydrophobic micelle core was found to be less crucial. Thus, hydrophobic mismatch appears to be less important in detergent micelles than in lipid bilayers and individual detergent molecules appear to be able to stretch within a micelle to match the hydrophobic thickness of the peptide. However, the stability of the GpA TM helix dimer linearly depends on the aggregation number of the lyso-PC detergents, indicating that not only is the chemistry of the detergent headgroup and acyl-chain region central for classifying a detergent as harsh or mild, but the detergent aggregation number might also be important.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Sequence-specific dimerization of a transmembrane helix in amphipol A8-35.PLoS One. 2014 Oct 27;9(10):e110970. doi: 10.1371/journal.pone.0110970. eCollection 2014. PLoS One. 2014. PMID: 25347769 Free PMC article.
-
Structure of a protein-detergent complex: the balance between detergent cohesion and binding.Eur Biophys J. 2011 Oct;40(10):1143-55. doi: 10.1007/s00249-011-0745-9. Epub 2011 Sep 8. Eur Biophys J. 2011. PMID: 21901295
-
Transmembrane helix-helix interactions: comparative simulations of the glycophorin a dimer.Biochemistry. 2006 Dec 5;45(48):14298-310. doi: 10.1021/bi0610911. Biochemistry. 2006. PMID: 17128969
-
Detergent-mediated protein aggregation.Chem Phys Lipids. 2013 Apr;169:72-84. doi: 10.1016/j.chemphyslip.2013.02.005. Epub 2013 Mar 4. Chem Phys Lipids. 2013. PMID: 23466535 Free PMC article. Review.
-
The mechanism of detergent solubilization of lipid bilayers.Biophys J. 2013 Jul 16;105(2):289-99. doi: 10.1016/j.bpj.2013.06.007. Biophys J. 2013. PMID: 23870250 Free PMC article. Review.
Cited by
-
Hydrophobic mismatch and sequence specificity compete when transmembrane helix-helix interactions are measured with the TOXCAT assay.Front Chem. 2022 Nov 28;10:1049310. doi: 10.3389/fchem.2022.1049310. eCollection 2022. Front Chem. 2022. PMID: 36518980 Free PMC article.
-
Sequence-specific dimerization of a transmembrane helix in amphipol A8-35.PLoS One. 2014 Oct 27;9(10):e110970. doi: 10.1371/journal.pone.0110970. eCollection 2014. PLoS One. 2014. PMID: 25347769 Free PMC article.
-
Fluorophores, environments, and quantification techniques in the analysis of transmembrane helix interaction using FRET.Biopolymers. 2015 Jul;104(4):247-64. doi: 10.1002/bip.22667. Biopolymers. 2015. PMID: 25968159 Free PMC article. Review.
-
Kinetics of Membrane Protein-Detergent Interactions Depend on Protein Electrostatics.J Phys Chem B. 2018 Oct 18;122(41):9471-9481. doi: 10.1021/acs.jpcb.8b07889. Epub 2018 Oct 5. J Phys Chem B. 2018. PMID: 30251852 Free PMC article.
-
Cysteine residues impact the stability and micelle interaction dynamics of the human mitochondrial β-barrel anion channel hVDAC-2.PLoS One. 2014 Mar 18;9(3):e92183. doi: 10.1371/journal.pone.0092183. eCollection 2014. PLoS One. 2014. PMID: 24642864 Free PMC article.
References
-
- Veerappan A., Cymer F., Schneider D. The tetrameric α-helical membrane protein GlpF unfolds via a dimeric folding intermediate. Biochemistry. 2011;50:10223–10230. - PubMed
-
- Melnyk R.A., Partridge A.W., Deber C.M. Retention of native-like oligomerization states in transmembrane segment peptides: application to the Escherichia coli aspartate receptor. Biochemistry. 2001;40:11106–11113. - PubMed
-
- Federkeil S.L., Winstone T.L., Turner R.J. Examination of EmrE conformational differences in various membrane mimetic environments. Biochem. Cell Biol. 2003;81:61–70. - PubMed
-
- Chou J.J., Kaufman J.D., Bax A. Micelle-induced curvature in a water-insoluble HIV-1 Env peptide revealed by NMR dipolar coupling measurement in stretched polyacrylamide gel. J. Am. Chem. Soc. 2002;124:2450–2451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

