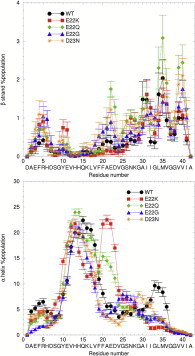
Effects of familial mutations on the monomer structure of Aβ₄₂
- PMID: 23260058
- PMCID: PMC3525840
- DOI: 10.1016/j.bpj.2012.11.009
Effects of familial mutations on the monomer structure of Aβ₄₂
Abstract
Amyloid beta (Aβ) peptide plays an important role in Alzheimer's disease. A number of mutations in the Aβ sequence lead to familial Alzheimer's disease, congophilic amyloid angiopathy, or hereditary cerebral hemorrhage with amyloid. Using molecular dynamics simulations of ∼200 μs for each system, we characterize and contrast the consequences of four pathogenic mutations (Italian, Dutch, Arctic, and Iowa) for the structural ensemble of the Aβ monomer. The four familial mutations are found to have distinct consequences for the monomer structure.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Investigating how peptide length and a pathogenic mutation modify the structural ensemble of amyloid beta monomer.Biophys J. 2012 Jan 18;102(2):315-24. doi: 10.1016/j.bpj.2011.12.002. Biophys J. 2012. PMID: 22339868 Free PMC article.
-
Structural heterogeneity in familial Alzheimer's disease mutants of amyloid-beta peptides.Mol Biosyst. 2013 May;9(5):997-1003. doi: 10.1039/c2mb25457c. Mol Biosyst. 2013. PMID: 23358498
-
Changes to the structure and dynamics in mutations of Aβ(21-30) caused by ions in solution.J Phys Chem B. 2013 Dec 5;117(48):14907-15. doi: 10.1021/jp408579v. Epub 2013 Nov 21. J Phys Chem B. 2013. PMID: 24261958
-
Dual effects of familial Alzheimer's disease mutations (D7H, D7N, and H6R) on amyloid β peptide: correlation dynamics and zinc binding.Proteins. 2014 Dec;82(12):3286-97. doi: 10.1002/prot.24669. Epub 2014 Oct 21. Proteins. 2014. PMID: 25137638
-
Exploring the Alzheimer amyloid-β peptide conformational ensemble: A review of molecular dynamics approaches.Peptides. 2015 Jul;69:86-91. doi: 10.1016/j.peptides.2015.04.009. Epub 2015 Apr 20. Peptides. 2015. PMID: 25908410 Review.
Cited by
-
Folding@home: achievements from over twenty years of citizen science herald the exascale era.ArXiv [Preprint]. 2023 Mar 15:arXiv:2303.08993v1. ArXiv. 2023. Update in: Biophys J. 2023 Jul 25;122(14):2852-2863. doi: 10.1016/j.bpj.2023.03.028. PMID: 36994157 Free PMC article. Updated. Preprint.
-
Two distinct β-sheet structures in Italian-mutant amyloid-beta fibrils: a potential link to different clinical phenotypes.Cell Mol Life Sci. 2015 Dec;72(24):4899-913. doi: 10.1007/s00018-015-1983-2. Epub 2015 Jul 21. Cell Mol Life Sci. 2015. PMID: 26190022 Free PMC article.
-
Emergence of Barrel Motif in Amyloid-β Trimer: A Computational Study.J Phys Chem B. 2020 Nov 25;124(47):10617-10631. doi: 10.1021/acs.jpcb.0c05508. Epub 2020 Nov 12. J Phys Chem B. 2020. PMID: 33180492 Free PMC article.
-
The Effects of Aβ1-42 Binding to the SARS-CoV-2 Spike Protein S1 Subunit and Angiotensin-Converting Enzyme 2.Int J Mol Sci. 2021 Jul 30;22(15):8226. doi: 10.3390/ijms22158226. Int J Mol Sci. 2021. PMID: 34360989 Free PMC article.
-
The OPEP protein model: from single molecules, amyloid formation, crowding and hydrodynamics to DNA/RNA systems.Chem Soc Rev. 2014 Jul 7;43(13):4871-93. doi: 10.1039/c4cs00048j. Epub 2014 Apr 23. Chem Soc Rev. 2014. PMID: 24759934 Free PMC article. Review.
References
-
- Lesné S., Koh M.T., Ashe K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. - PubMed
-
- Selkoe D.J., Podlisny M.B. Deciphering the genetic basis of Alzheimer’s disease. Annu. Rev. Genomics Hum. Genet. 2002;3:67–99. - PubMed
-
- Murakami K., Irie K., Shirasawa T. Neurotoxicity and physicochemical properties of Aβ mutant peptides from cerebral amyloid angiopathy. J. Biol. Chem. 2003;278:46179–46187. - PubMed
-
- Nilsberth C., Westlind-Danielsson A., Lannfelt L. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat. Neurosci. 2001;4:887–893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases