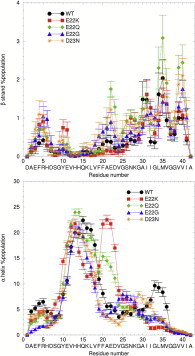
Effects of familial mutations on the monomer structure of Aβ₄₂
- PMID: 23260058
- PMCID: PMC3525840
- DOI: 10.1016/j.bpj.2012.11.009
Effects of familial mutations on the monomer structure of Aβ₄₂
Abstract
Amyloid beta (Aβ) peptide plays an important role in Alzheimer's disease. A number of mutations in the Aβ sequence lead to familial Alzheimer's disease, congophilic amyloid angiopathy, or hereditary cerebral hemorrhage with amyloid. Using molecular dynamics simulations of ∼200 μs for each system, we characterize and contrast the consequences of four pathogenic mutations (Italian, Dutch, Arctic, and Iowa) for the structural ensemble of the Aβ monomer. The four familial mutations are found to have distinct consequences for the monomer structure.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Lesné S., Koh M.T., Ashe K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. - PubMed
-
- Selkoe D.J., Podlisny M.B. Deciphering the genetic basis of Alzheimer’s disease. Annu. Rev. Genomics Hum. Genet. 2002;3:67–99. - PubMed
-
- Murakami K., Irie K., Shirasawa T. Neurotoxicity and physicochemical properties of Aβ mutant peptides from cerebral amyloid angiopathy. J. Biol. Chem. 2003;278:46179–46187. - PubMed
-
- Nilsberth C., Westlind-Danielsson A., Lannfelt L. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat. Neurosci. 2001;4:887–893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases