Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer
- PMID: 23260083
- PMCID: PMC3709838
- DOI: 10.1016/j.jcyt.2012.10.003
Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer
Abstract
Background aims: Many ovarian cancers originate from ovarian surface epithelium, where they develop from cysts intermixed with stroma. The stromal layer is critical to the progression and survival of the neoplasm and consequently is recruited into the tumor microenvironment.
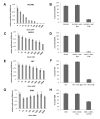
Methods: Using both syngeneic mouse tumors (ID8-R) and human xenograft (OVCAR3, SKOV3) tumor models, we first confirmed that intraperitoneally injected circulating mesenchymal stem cells (MSCs) could target, preferentially engraft and differentiate into α-smooth muscle actin-positive myofibroblasts, suggesting their role as "reactive stroma" in ovarian carcinoma development and confirming their potential as a targeted delivery vehicle for the intratumoral production of interferon-β (IFN-β). Mice with ovarian carcinomas then received weekly intraperitoneal injections of IFN-β expressing MSCs.
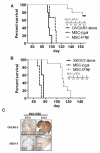
Results: Intraperitoneal injections of IFN-β expressing MSCs resulted in complete eradication of tumors in 70% of treated OVCAR3 mice (P = 0.004) and an increased survival of treated SKOV3 mice compared with controls (P = 0.01). Similar tumor growth control was observed using murine IFN-β delivered by murine MSCs in ID8-R ovarian carcinoma. As a potential mechanism of tumor killing, MSCs produced IFN-β-induced caspase-dependent tumor cell apoptosis.
Conclusions: Our results demonstrate that ovarian carcinoma engrafts MSCs to participate in myofibrovascular networks and that IFN-β produced by MSCs intratumorally modulates tumor kinetics, resulting in prolonged survival.
Published by Elsevier Inc.
Figures



Similar articles
-
Effects of mesenchymal stem cells harboring the Interferon-β gene on A549 lung cancer in nude mice.Pathol Res Pract. 2019 Mar;215(3):586-593. doi: 10.1016/j.prp.2019.01.013. Epub 2019 Jan 14. Pathol Res Pract. 2019. PMID: 30683475
-
[Human umbilical cord mesenchymal stem cells with adenovirus-mediated interleukin 12 gene transduction inhibits the growth of ovarian carcinoma cells both in vitro and in vivo].Nan Fang Yi Ke Da Xue Xue Bao. 2011 May;31(5):903-7. Nan Fang Yi Ke Da Xue Xue Bao. 2011. PMID: 21602155 Chinese.
-
Mesenchymal stem cells modified to express interferon-β inhibit the growth of prostate cancer in a mouse model.J Int Med Res. 2012;40(1):317-27. doi: 10.1177/147323001204000132. J Int Med Res. 2012. PMID: 22429371
-
Gene therapy of ovarian cancer using IL-21-secreting human umbilical cord mesenchymal stem cells in nude mice.J Ovarian Res. 2014 Jan 20;7:8. doi: 10.1186/1757-2215-7-8. J Ovarian Res. 2014. PMID: 24444073 Free PMC article.
-
An Orthotopic Mouse Model of Ovarian Cancer using Human Stroma to Promote Metastasis.J Vis Exp. 2021 Mar 23;(169). doi: 10.3791/62382. J Vis Exp. 2021. PMID: 33843939
Cited by
-
Engineering Stem Cells for Biomedical Applications.Adv Healthc Mater. 2016 Jan 7;5(1):10-55. doi: 10.1002/adhm.201400842. Epub 2015 Mar 13. Adv Healthc Mater. 2016. PMID: 25772134 Free PMC article. Review.
-
Bioengineered adipose-derived stem cells for targeted enzyme-prodrug therapy of ovarian cancer intraperitoneal metastasis.J Control Release. 2019 Oct;311-312:273-287. doi: 10.1016/j.jconrel.2019.09.006. Epub 2019 Sep 6. J Control Release. 2019. PMID: 31499084 Free PMC article.
-
Theoretical basis, state and challenges of living cell-based drug delivery systems.Theranostics. 2024 Aug 19;14(13):5152-5183. doi: 10.7150/thno.99257. eCollection 2024. Theranostics. 2024. PMID: 39267776 Free PMC article. Review.
-
Bacteria and cells as alternative nano-carriers for biomedical applications.Expert Opin Drug Deliv. 2022 Jan;19(1):103-118. doi: 10.1080/17425247.2022.2029844. Epub 2022 Jan 25. Expert Opin Drug Deliv. 2022. PMID: 35076351 Free PMC article. Review.
-
Interplay between mesenchymal stem cell and tumor and potential application.Hum Cell. 2020 Jul;33(3):444-458. doi: 10.1007/s13577-020-00369-z. Epub 2020 May 6. Hum Cell. 2020. PMID: 32378164 Review.
References
-
- American Cancer SocietyCancer Facts & Figures 2009. American Cancer Society; Atlanta: 2009.
-
- Ghahremani M, Dorrington FoghiA. JHEtiology of ovarian cancer: A proposed mechanism. Med.Hypotheses. 1999;52:23–26. - PubMed
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA-16672/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- CA-1094551/CA/NCI NIH HHS/United States
- R01 CA109451/CA/NCI NIH HHS/United States
- CA-49639/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- R01CA109451/CA/NCI NIH HHS/United States
- CA-116199/CA/NCI NIH HHS/United States
- CA-55164/CA/NCI NIH HHS/United States
- P01 CA055164/CA/NCI NIH HHS/United States
- RC1 CA146381/CA/NCI NIH HHS/United States
- RC1CA146381/CA/NCI NIH HHS/United States
- P30 CA012197/CA/NCI NIH HHS/United States
- P01 CA049639/CA/NCI NIH HHS/United States
- R01 NS069964/NS/NINDS NIH HHS/United States
- P50CA083639/CA/NCI NIH HHS/United States
- R01NS06994/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

