Assay validation for the assessment of adipogenesis of multipotential stromal cells--a direct comparison of four different methods
- PMID: 23260089
- PMCID: PMC3539160
- DOI: 10.1016/j.jcyt.2012.07.001
Assay validation for the assessment of adipogenesis of multipotential stromal cells--a direct comparison of four different methods
Abstract
Background aims: Mesenchymal stromal cells (MSCs) are regenerative and immuno-privileged cells that are used for both tissue regeneration and treatment of severe inflammation-related disease. For quality control of manufactured MSC batches in regard to mature fat cell contamination, a quantitative method for measuring adipogenesis is needed.
Methods: Four previously proposed methods were validated with the use of bone marrow (BM) MSCs during a 21-day in vitro assay. Oil red staining was scored semiquantitatively; peroxisome proliferator activated receptor-γ and fatty acid binding protein (FABP)4 transcripts were measured by quantitative real-time polymerase chain reaction; FABP4 protein accumulation was evaluated by flow cytometry; and Nile red/4',6-diamidino-2-phenylindole (DAPI) ratios were measured in fluorescent microplate assay. Skin fibroblasts and MSCs from fat pad, cartilage and umbilical cord were used as controls.
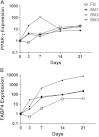
Results: Oil red staining indicated considerable heterogeneity between BM donors and individual cells within the same culture. FABP4 transcript levels increased 100- to 5000-fold by day 21, with large donor variability observed. Flow cytometry revealed increasing intra-culture heterogeneity over time; more granular cells accumulated more FABP4 protein and Nile red fluorescence compared with less granular cells. Nile red increase in day-21 MSCs was ~5- and 4-fold, measured by flow cytometry or microplate assay, respectively. MSC proliferation/apoptosis was accounted through the use of Nile red/DAPI ratios; adipogenesis levels in day-21 BM MSCs increased ~13-fold, with significant correlations with oil red scoring observed for MSC from other sources.
Conclusions: Flow cytometry permits the study of MSC differentiation at the single-cell level and sorting more and less mature cells from mixed cell populations. The microplate assay with the use of the Nile red/DAPI ratio provides rapid quantitative measurements and could be used as a low-cost, high-throughput method to quality-control MSC batches from different tissue sources.
Copyright © 2013 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Gene expression pattern of human chorion-derived mesenchymal stem cells during adipogenic differentiation.Yonsei Med J. 2012 Sep;53(5):1036-44. doi: 10.3349/ymj.2012.53.5.1036. Yonsei Med J. 2012. PMID: 22869490 Free PMC article.
-
IL-17A and TNF Modulate Normal Human Spinal Entheseal Bone and Soft Tissue Mesenchymal Stem Cell Osteogenesis, Adipogenesis, and Stromal Function.Cells. 2021 Feb 6;10(2):341. doi: 10.3390/cells10020341. Cells. 2021. PMID: 33562025 Free PMC article.
-
Automated microscopy as a quantitative method to measure differences in adipogenic differentiation in preparations of human mesenchymal stromal cells.Cytotherapy. 2013 Dec;15(12):1527-40. doi: 10.1016/j.jcyt.2013.04.010. Epub 2013 Aug 29. Cytotherapy. 2013. PMID: 23992827 Free PMC article.
-
Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton's jelly and bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2017 Apr 26;8(1):102. doi: 10.1186/s13287-017-0555-9. Stem Cell Res Ther. 2017. PMID: 28446235 Free PMC article.
-
The human arthritic hip joint is a source of mesenchymal stromal cells (MSCs) with extensive multipotent differentiation potential.BMC Musculoskelet Disord. 2020 May 13;21(1):297. doi: 10.1186/s12891-020-03340-z. BMC Musculoskelet Disord. 2020. PMID: 32404085 Free PMC article.
Cited by
-
Impact of source tissue and ex vivo expansion on the characterization of goat mesenchymal stem cells.J Anim Sci Biotechnol. 2015 Jan 11;6(1):1. doi: 10.1186/2049-1891-6-1. eCollection 2015. J Anim Sci Biotechnol. 2015. PMID: 25838897 Free PMC article.
-
Simulated Microgravity Condition Alters the Gene Expression of some ECM and Adhesion Molecules in Adipose Derived Stem Cells.Int J Mol Cell Med. 2018 Summer;7(3):146-157. doi: 10.22088/IJMCM.BUMS.7.3.146. Epub 2018 Oct 8. Int J Mol Cell Med. 2018. PMID: 31565646 Free PMC article.
-
Reference Gene Expression in Adipose-Derived Stromal Cells Undergoing Adipogenic Differentiation.Tissue Eng Part C Methods. 2019 Jun;25(6):353-366. doi: 10.1089/ten.TEC.2019.0076. Tissue Eng Part C Methods. 2019. PMID: 31062665 Free PMC article.
-
Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect.PLoS One. 2013 Oct 14;8(10):e77481. doi: 10.1371/journal.pone.0077481. eCollection 2013. PLoS One. 2013. PMID: 24155963 Free PMC article.
-
High-throughput, nonperturbing quantification of lipid droplets with digital holographic microscopy.J Lipid Res. 2018 Jul;59(7):1301-1310. doi: 10.1194/jlr.D085217. Epub 2018 Apr 5. J Lipid Res. 2018. PMID: 29622579 Free PMC article.
References
-
- Barry F.P., Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. - PubMed
-
- McGonagle D., Jones E. A potential role for synovial fluid mesenchymal stem cells in ligament regeneration. Rheumatology. 2008 - PubMed
-
- Pittenger MF., Mackay AM., Beck SC., Jaiswal RK., Douglas R., Mosca JD. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Jones EA., Kinsey SE., English A., Jones RA., Straszynski L., Meredith DM. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

