Cloning and expression of feline colony stimulating factor receptor (CSF-1R) and analysis of the species specificity of stimulation by colony stimulating factor-1 (CSF-1) and interleukin-34 (IL-34)
- PMID: 23260168
- PMCID: PMC3573236
- DOI: 10.1016/j.cyto.2012.11.014
Cloning and expression of feline colony stimulating factor receptor (CSF-1R) and analysis of the species specificity of stimulation by colony stimulating factor-1 (CSF-1) and interleukin-34 (IL-34)
Abstract
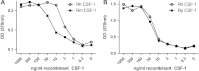
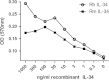
Colony stimulating factor (CSF-1) and its receptor, CSF-1R, have been previously well studied in humans and rodents to dissect the role they play in development of cells of the mononuclear phagocyte system. A second ligand for the CSF-1R, IL-34 has been described in several species. In this study, we have cloned and expressed the feline CSF-1R and examined the responsiveness to CSF-1 and IL-34 from a range of species. The results indicate that pig and human CSF-1 and human IL-34 are equally effective in cats, where both mouse CSF-1 and IL-34 are significantly less active. Recombinant human CSF-1 can be used to generate populations of feline bone marrow and monocyte derived macrophages that can be used to further dissect macrophage-specific gene expression in this species, and to compare it to data derived from mouse, human and pig. These results set the scene for therapeutic use of CSF-1 and IL-34 in cats.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





References
-
- Hume D.A., Macdonald K.P. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. - PubMed
-
- Gow D.J., Garceau V., Kapetanovic R., Sester D.P., Fici G.J., Shelly J.A. Cloning and Expression of Porcine Colony Stimulating Factor-1 (CSF-1) and Colony Stimulating Factor-1 Receptor (CSF-1R) and analysis of the species specificity of stimulation by CSF-1 and Interleukin 34. Cytokine. 2012 http://dx.doi.org/10.1016/j.cyto.2012.08.008. - PMC - PubMed
-
- Chitu V., Stanley E.R. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. - PubMed
-
- Bonifer C., Hume D.A. The transcriptional regulation of the colony-stimulating factor 1 receptor (csf1r) gene during hematopoiesis. Front. Biosci. 2008;13:549–560. - PubMed
-
- Sherr C.J., Rettenmier C.W., Sacca R., Roussel M.F., Look A.T., Stanley E.R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985;41:665–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/G004013/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/20251969/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0901193/MRC_/Medical Research Council/United Kingdom
- 338BCB R40954/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

