A multilevel intervention to increase community hospital use of alteplase for acute stroke (INSTINCT): a cluster-randomised controlled trial
- PMID: 23260188
- PMCID: PMC3939784
- DOI: 10.1016/S1474-4422(12)70311-3
A multilevel intervention to increase community hospital use of alteplase for acute stroke (INSTINCT): a cluster-randomised controlled trial
Abstract
Background: Use of alteplase improves outcome in some patients with stroke. Several types of barrier frequently prevent its use. We assessed whether a standardised, barrier-assessment, multicomponent intervention could increase alteplase use in community hospitals in Michigan, USA.
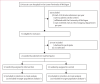
Methods: In a cluster-randomised controlled trial, we selected adult, non-specialty, acute-care community hospitals in the Lower Peninsula of Michigan, USA. Eligible hospitals discharged at least 100 patients who had had a stroke per year, had less than 100 000 visits to the emergency department per year, and were not academic comprehensive stroke centres. Using a computer-generated randomisation sequence, we selected 12 matched pairs of eligible hospitals. Within pairs, the hospitals were allocated to intervention or control groups with restricted randomisation in January, 2007. Between January, 2007, and December, 2007, intervention hospitals implemented a multicomponent intervention that included qualitative and quantitative assessment of barriers to alteplase use and ways to address the findings, and provided additional support. The primary outcome was change in alteplase use in patients with stroke in emergency departments between the pre-intervention period (January, 2005, to December, 2006) and the post-intervention period (January, 2008, to January, 2010). Physicians in participating hospitals and the coordinating centre could not be masked to group assignment, but were masked to progress made in paired control hospitals. External medical reviewers who were masked to group assignment assessed outcomes. We did intention-to-treat (ITT) and target-population (without one pair that was excluded after randomisation) analyses. This trial is registered at ClinicalTrials.gov, number NCT00349479.
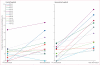
Findings: All 24 hospitals completed the study. Overall, 745 of 40 823 patients with stroke received intravenous alteplase treatment. In the ITT analysis, the proportion of patients with stroke who were admitted and treated with alteplase increased between the pre-intervention and post-intervention periods in intervention hospitals (89 [1·25%] of 7119 patients to 235 [2·79%] of 8419) to a greater extent than in control hospitals (99 [1·25%] of 7946 to 194 [2·10%] of 9222), but the difference between groups was not significant (relative risk [RR] 1·37, 95% CI 0·96-1·93; p=0·08). In the target-population analysis, the increase in alteplase use in intervention hospitals (59 [1·00%] of 5882 to 191 [2·62%] of 7288) was significantly greater than in control hospitals (65 [1·09%] of 5957 to 120 [1·72%] of 6989; RR 1·68, 95% CI 1·09-2·57; p=0·02), but was still clinically modest.
Interpretation: The intervention did not significantly increase alteplase use in patients with ischaemic stroke. The increase in use of alteplase in the target population was significant, but smaller than the effect to which the study was powered. Additional strategies to increase acute stroke treatment are needed.
Funding: National Institutes of Health National Institute of Neurological Disorders and Stroke.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Implementation of thrombolysis for ischaemic stroke.Lancet Neurol. 2013 Feb;12(2):120-1. doi: 10.1016/S1474-4422(12)70304-6. Epub 2012 Dec 21. Lancet Neurol. 2013. PMID: 23260190 No abstract available.
References
-
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. - PubMed
-
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–87. - PubMed
-
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. - PubMed
-
- Hills NK, Johnston SC. Why are eligible thrombolysis candidates left untreated? Am J Prev Med. 2006;31(suppl 2):S210–16. - PubMed
-
- Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use. Stroke. 2008;39:924–28. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

