A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine
- PMID: 23260506
- PMCID: PMC3621718
- DOI: 10.1016/j.amjmed.2012.07.005
A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine
Abstract
Objective: To determine the impact of cognitive behavioral therapy on outcomes in primary care, office-based buprenorphine/naloxone treatment of opioid dependence.
Methods: We conducted a 24-week randomized clinical trial in 141 opioid-dependent patients in a primary care clinic. Patients were randomized to physician management or physician management plus cognitive behavioral therapy. Physician management was brief, manual guided, and medically focused; cognitive behavioral therapy was manual guided and provided for the first 12 weeks of treatment. The primary outcome measures were self-reported frequency of illicit opioid use and the maximum number of consecutive weeks of abstinence from illicit opioids, as documented by urine toxicology and self-report.
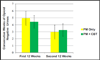
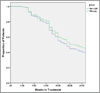
Results: The 2 treatments had similar effectiveness with respect to reduction in the mean self-reported frequency of opioid use, from 5.3 days per week (95% confidence interval, 5.1-5.5) at baseline to 0.4 (95% confidence interval, 0.1-0.6) for the second half of maintenance (P<.001 for the comparisons of induction and maintenance with baseline), with no differences between the 2 groups (P=.96) or between the treatments over time (P=.44). For the maximum consecutive weeks of opioid abstinence there was a significant main effect of time (P<.001), but the interaction (P=.11) and main effect of group (P=.84) were not significant. No differences were observed on the basis of treatment assignment with respect to cocaine use or study completion.
Conclusions: Among patients receiving buprenorphine/naloxone in primary care for opioid dependence, the effectiveness of physician management did not differ significantly from that of physician management plus cognitive behavioral therapy.
Trial registration: ClinicalTrials.gov NCT00632151.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Primary care management of opioid dependence: the addition of CBT gives no extra benefit compared to standard physician management alone.Evid Based Ment Health. 2013 Aug;16(3):76. doi: 10.1136/eb-2013-101253. Epub 2013 Apr 24. Evid Based Ment Health. 2013. PMID: 23616210 No abstract available.
References
-
- Anonymous. The DASIS Report: Facilities Providing Methadone/LAAM Treatment to Clients with Opiate Addiction. Rockville, MD: Substance Abuse Mental Health Services Administration, Office of Applied Studies; 2002.
-
- Anonymous. CESAR FAX. Vol 20. College Park, MD: University of Maryland; 2011. Buprenorphine Treatment for Opioid Dependence; p. 34.
-
- Anonymous. The N-SSATS Report: Overview of Opioid Treatment Programs Within the United States: 2008. Rockville, MD: Substance Abuse Mental Health and Services Administration, Office of Applied Studies; 2010.
-
- Kissin W, McLeod C, Sonnefeld J, Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. J Addict Dis. 2006;25:91–103. - PubMed
-
- Arfken CL, Johanson C-E, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: national surveys of physicians. J Subst Abuse Treat. 2010;39:96–104. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

