MORC2 signaling integrates phosphorylation-dependent, ATPase-coupled chromatin remodeling during the DNA damage response
- PMID: 23260667
- PMCID: PMC3554793
- DOI: 10.1016/j.celrep.2012.11.018
MORC2 signaling integrates phosphorylation-dependent, ATPase-coupled chromatin remodeling during the DNA damage response
Abstract
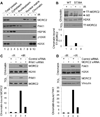
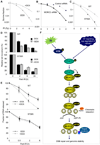
Chromatin dynamics play a central role in maintaining genome integrity, but how this is achieved remains largely unknown. Here, we report that microrchidia CW-type zinc finger 2 (MORC2), an uncharacterized protein with a derived PHD finger domain and a conserved GHKL-type ATPase module, is a physiological substrate of p21-activated kinase 1 (PAK1), an important integrator of extracellular signals and nuclear processes. Following DNA damage, MORC2 is phosphorylated on serine 739 in a PAK1-dependent manner, and phosphorylated MORC2 regulates its DNA-dependent ATPase activity to facilitate chromatin remodeling. Moreover, MORC2 associates with chromatin and promotes gamma-H2AX induction in a PAK1 phosphorylation-dependent manner. Consequently, cells expressing MORC2-S739A mutation displayed a reduction in DNA repair efficiency and were hypersensitive to DNA-damaging agent. These findings suggest that the PAK1-MORC2 axis is critical for orchestrating the interplay between chromatin dynamics and the maintenance of genomic integrity through sequentially integrating multiple essential enzymatic processes.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

