The effect of the acid-sensitivity of 4-(N)-stearoyl gemcitabine-loaded micelles on drug resistance caused by RRM1 overexpression
- PMID: 23261218
- PMCID: PMC3552003
- DOI: 10.1016/j.biomaterials.2012.11.053
The effect of the acid-sensitivity of 4-(N)-stearoyl gemcitabine-loaded micelles on drug resistance caused by RRM1 overexpression
Abstract
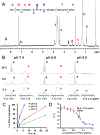
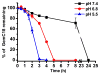
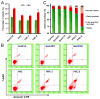
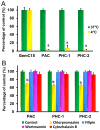
Chemoresistance is a major issue for most gemcitabine-related chemotherapies. The overexpression of ribonucleotide reductase subunit M1 (RRM1) plays a key role in gemcitabine resistance. In this study, we synthesized a new highly acid-sensitive amphiphilic micelle material by conjugating hydrophilic polyethylene glycol with a hydrophobic stearic acid derivative (C18) using a hydrazone bond, which was named as PHC-2. A lipophilic prodrug of gemcitabine, 4-(N)-stearoyl gemcitabine (GemC18), was loaded into micelles prepared with PHC-2, a previously synthesized less acid-sensitive PHC-1, and their acid-insensitive counterpart, PAC. GemC18 loaded in acid-sensitive micelles can overcome gemcitabine resistance, and GemC18 in the highly acid-sensitive PHC-2 micelles was more cytotoxic than in the less acid-sensitive PHC-1 micelles. Mechanistic studies revealed that upon cellular uptake and lysosomal delivery, GemC18 in the acid-sensitive micelles was released and hydrolyzed more efficiently. Furthermore, GemC18 loaded in the highly acid-sensitive PHC-2 micelles inhibited the expression of RRM1 and increased the level of gemcitabine triphosphate (dFdCTP) in gemcitabine resistant tumor cells. The strategy of delivering lipophilized nucleoside analogs using highly acid-sensitive micelles may represent a new platform technology to increase the antitumor activity of nucleoside analogs and to overcome tumor cell resistance to them.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Barton-Burke M. Gemcitabine: a pharmacologic and clinical overview. Cancer Nurs. 1999;22:176–83. - PubMed
-
- Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, et al. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58:4349–57. - PubMed
-
- Bouffard DY, Laliberte J, Momparler RL. Kinetic studies on 2′,2′-difluorodeoxycytidine (Gemcitabine) with purified human deoxycytidine kinase and cytidine deaminase. Biochem Pharmacol. 1993;45:1857–61. - PubMed
-
- Plunkett W, Huang P, Gandhi V. Preclinical characteristics of gemcitabine. Anticancer Drugs. 1995;6 (Suppl 6):7–13. - PubMed
-
- Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

