Molecular basis of in vivo biofilm formation by bacterial pathogens
- PMID: 23261595
- PMCID: PMC3530155
- DOI: 10.1016/j.chembiol.2012.10.022
Molecular basis of in vivo biofilm formation by bacterial pathogens
Abstract
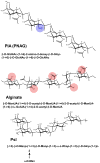
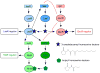
Bacterial biofilms are involved in a multitude of serious chronic infections. In recent years, modeling of biofilm infection in vitro has led to the identification of microbial determinants that govern biofilm development. However, we lack information as to whether the biofilm formation mechanisms identified in vitro have relevance for biofilm-associated infection. Here, we discuss the molecular basis of biofilm formation. Staphylococci and Pseudomonas aeruginosa are used to illustrate key points because their biofilm development process has been well studied. We focus on in vivo findings, such as obtained in animal infection models, and critically evaluate the in vivo relevance of in vitro findings. Although conflicting results about the role of quorum sensing in biofilm formation have been obtained, we argue that integration of in vitro and in vivo studies allows a differentiated view of this mechanism as it relates to biofilm infection.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

