The effects of jaspamide on human cardiomyocyte function and cardiac ion channel activity
- PMID: 23261645
- PMCID: PMC3587659
- DOI: 10.1016/j.tiv.2012.12.005
The effects of jaspamide on human cardiomyocyte function and cardiac ion channel activity
Abstract
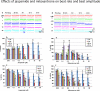
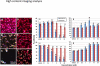
Jaspamide (jasplakinolide; NSC-613009) is a cyclodepsipeptide that has antitumor activity. A narrow margin of safety was observed between doses required for efficacy in mouse tumor models and doses that caused severe acute toxicity in rats and dogs. We explored the hypothesis that the observed toxicity was due to cardiotoxicity. Jaspamide was tested in a patch clamp assay to determine its effect on selected cardiac ion channels. Jaspamide (10 μM) inhibited Kv1.5 activity by 98.5%. Jaspamide also inhibited other channels including Cav1.2, Cav3.2, and HCN2; however, the Kv11.1 (hERG) channel was minimally affected. Using spontaneously contracting human cardiomyocytes derived from induced pluripotent stem cells, effects on cardiomyocyte contraction and viability were also examined. Jaspamide (30 nM to 30 μM) decreased cardiomyocyte cell indices and beat amplitude, putative measurements of cell viability and cardiac contractility, respectively. Concentration-dependent increases in rhythmic beating rate were noted at ≤ 6 h of treatment, followed by dose-dependent decreases after 6 and 72 h exposure. The toxic effects of jaspamide were compared with that of the known cardiotoxicant mitoxantrone, and confirmed by multiparameter fluorescence imaging analysis. These results support the hypothesis that the toxicity observed in rats and dogs is due to toxic effects of jaspamide on cardiomyocytes.
Published by Elsevier Ltd.
Figures


Similar articles
-
Cardiac safety assessment with motion field imaging analysis of human iPS cell-derived cardiomyocytes is improved by an integrated evaluation with cardiac ion channel profiling.J Toxicol Sci. 2019;44(12):859-870. doi: 10.2131/jts.44.859. J Toxicol Sci. 2019. PMID: 31813905
-
Evaluation of nefazodone-induced cardiotoxicity in human induced pluripotent stem cell-derived cardiomyocytes.Toxicol Appl Pharmacol. 2016 Apr 1;296:42-53. doi: 10.1016/j.taap.2016.01.015. Epub 2016 Jan 25. Toxicol Appl Pharmacol. 2016. PMID: 26821276
-
Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes.Toxicol Appl Pharmacol. 2013 Oct 1;272(1):245-55. doi: 10.1016/j.taap.2013.04.027. Epub 2013 May 21. Toxicol Appl Pharmacol. 2013. PMID: 23707608
-
Finding the rhythm of sudden cardiac death: new opportunities using induced pluripotent stem cell-derived cardiomyocytes.Circ Res. 2015 Jun 5;116(12):1989-2004. doi: 10.1161/CIRCRESAHA.116.304494. Circ Res. 2015. PMID: 26044252 Free PMC article. Review.
-
Evolution of strategies to improve preclinical cardiac safety testing.Nat Rev Drug Discov. 2016 Jul;15(7):457-71. doi: 10.1038/nrd.2015.34. Epub 2016 Feb 19. Nat Rev Drug Discov. 2016. PMID: 26893184 Review.
Cited by
-
Cytoskeletal Dynamics in Epithelial-Mesenchymal Transition: Insights into Therapeutic Targets for Cancer Metastasis.Cancers (Basel). 2021 Apr 14;13(8):1882. doi: 10.3390/cancers13081882. Cancers (Basel). 2021. PMID: 33919917 Free PMC article. Review.
-
Bioactivity of Marine-Derived Peptides and Proteins: A Review.Mar Drugs. 2025 Apr 4;23(4):157. doi: 10.3390/md23040157. Mar Drugs. 2025. PMID: 40278278 Free PMC article. Review.
-
Use of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs) to Monitor Compound Effects on Cardiac Myocyte Signaling Pathways.Curr Protoc Chem Biol. 2015 Sep 1;7(3):141-185. doi: 10.1002/9780470559277.ch150035. Curr Protoc Chem Biol. 2015. PMID: 26331525 Free PMC article.
-
Jaspamide/Jasplakinolide Is Synthesized by Jaspinella (Tectomicrobia) Bacteria in Sponges.J Nat Prod. 2025 Jun 27;88(6):1471-1480. doi: 10.1021/acs.jnatprod.5c00433. Epub 2025 Jun 4. J Nat Prod. 2025. PMID: 40468714
-
Multiomic Approach for Bioprospection: Investigation of Toxins and Peptides of Brazilian Sea Anemone Bunodosoma caissarum.Mar Drugs. 2023 Mar 22;21(3):197. doi: 10.3390/md21030197. Mar Drugs. 2023. PMID: 36976246 Free PMC article.
References
-
- Bachmann A, Gutcher I, Kopp K, Brendel J, Bosch RF, Busch AE, Gögelein H. Characterization of a novel Kv1.5 channel blocker in Xenopus oocytes, CHO cells, human and rat cardiomyocytes. Naunyn Schmiedebergs Arch. Pharmacol. 2001;364:472–478. - PubMed
-
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KLK, Korn E. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 1994;269:14869–14871. - PubMed
-
- Bubley G, Balk S. Treatment of androgen-independent prostate cancer. Oncologist. 1996;1:30. - PubMed
-
- Crews P, Manes LV, Boehler M. Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis sp. Tetrahedron Lett. 1986;27:2797–2800.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

