Effect of RGD functionalization and stiffness modulation of polyelectrolyte multilayer films on muscle cell differentiation
- PMID: 23261924
- PMCID: PMC4112381
- DOI: 10.1016/j.actbio.2012.12.015
Effect of RGD functionalization and stiffness modulation of polyelectrolyte multilayer films on muscle cell differentiation
Abstract
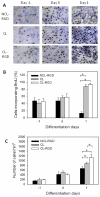
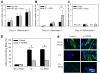
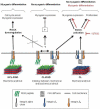
Skeletal muscle tissue engineering holds promise for the replacement of muscle damaged by injury and for the treatment of muscle diseases. Although arginylglycylaspartic acid (RGD) substrates have been widely explored in tissue engineering, there have been no studies aimed at investigating the combined effects of RGD nanoscale presentation and matrix stiffness on myogenesis. In the present work we use polyelectrolyte multilayer films made of poly(L-lysine) (PLL) and poly(L-glutamic) acid (PGA) as substrates of tunable stiffness that can be functionalized by a RGD adhesive peptide to investigate important events in myogenesis, including adhesion, migration, proliferation and differentiation. C2C12 myoblasts were used as cellular models. RGD presentation on soft films and increasing film stiffness could both induce cell adhesion, but the integrins involved in adhesion were different in the case of soft and stiff films. Soft films with RGD peptide appeared to be the most appropriate substrate for myogenic differentiation, while the stiff PLL/PGA films induced significant cell migration and proliferation and inhibited myogenic differentiation. ROCK kinase was found to be involved in the myoblast response to the different films. Indeed, its inhibition was sufficient to rescue differentiation on stiff films, but no significant changes were observed on stiff films with the RGD peptide. These results suggest that different signaling pathways may be activated depending on the mechanical and biochemical properties of multilayer films. This study emphasizes the advantage of soft PLL/PGA films presenting the RGD peptide in terms of myogenic differentiation. This soft RGD-presenting film may be further used as a coating of various polymeric scaffolds for muscle tissue engineering.
Copyright © 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





References
-
- Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat Rec. 1975;182:215–35. - PubMed
-
- Le Ricousse-Roussanne S, Larghero J, Zini JM, Barateau V, Foubert P, Uzan G, et al. Ex vivo generation of mature and functional human smooth muscle cells differentiated from skeletal myoblasts. Exp Cell Res. 2007;313:1337–46. - PubMed
-
- Schindeler A, Liu R, Little DG. The contribution of different cell lineages to bone repair: exploring a role for muscle stem cells. Differentiation. 2009;77:12–8. - PubMed
-
- Teboul L, Gaillard D, Staccini L, Inadera H, Amri EZ, Grimaldi PA. Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. J Biol Chem. 1995;270:28183–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

