Measles virus nucleocapsid protein, a key contributor to Paget's disease, increases IL-6 expression via down-regulation of FoxO3/Sirt1 signaling
- PMID: 23262029
- PMCID: PMC3552041
- DOI: 10.1016/j.bone.2012.12.007
Measles virus nucleocapsid protein, a key contributor to Paget's disease, increases IL-6 expression via down-regulation of FoxO3/Sirt1 signaling
Abstract
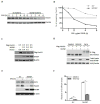
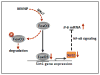
Measles virus plays an important role as an environmental factor in the pathogenesis of Paget's disease (PD). Previous studies have shown that IL-6 is increased in the bone marrow of Paget's patients and that measles virus nucleocapsid protein (MVNP) induces IL-6 secretion by pagetic osteoclasts. Further, IL-6 plays a critical role in the development of pagetic osteoclasts and bone lesions induced by PD, but the mechanisms regulating IL-6 production by MVNP remain unclear. Our current studies revealed that MVNP expression in osteoclast precursors down-regulated Sirt1 mRNA and protein, a negative regulator of NF-κB activity, which is a key factor for IL-6 expression. MVNP expression in NIH3T3 cells also elevated Il-6 transcription and impaired the expression of Sirt1 mRNA both under basal conditions and upon activation of the Sirt1 upstream regulator FoxO3 by LY294002 (a PI3K/AKT inhibitor). Luciferase activity assays showed that constitutively active FoxO3 abolished the repressive effect of MVNP on reporters driven by either FoxO3 response elements or the Sirt1 promoter. Further, protein stability assays revealed that FoxO3 was degraded more rapidly in MVNP-expressing cells than in control cells following the addition of cycloheximide. Similarly, co-transfection of MVNP and FoxO3 into HEK293 cells demonstrated that MVNP decreased the protein levels of over-expressed FoxO3 in a dose-dependent manner. Treatment with the proteasome inhibitor, MG132, blocked the MVNP-triggered decrease of FoxO3, and the treatment with the serine/threonine phosphatase inhibitor, calyculin A, revealed that MVNP increased phosphorylation of FoxO3. Further, over-expression of Sirt1 or treatment with the Sirt1 activator resveratrol blocked the increase in Il-6 transcription by MVNP. Finally, resveratrol reduced the numbers of TRAP positive multi-nuclear cells in bone marrow cultures from TRAP-MVNP transgenic mice to wild type levels. These results indicate that MVNP decreases FoxO3/Sirt1 signaling to enhance the levels of IL-6, which in part mediate MVNP's contribution to the development of Paget's disease.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
TBK1 mediates critical effects of measles virus nucleocapsid protein (MVNP) on pagetic osteoclast formation.J Bone Miner Res. 2014 Jan;29(1):90-102. doi: 10.1002/jbmr.2026. J Bone Miner Res. 2014. PMID: 23794264 Free PMC article.
-
Increased IL-6 expression in osteoclasts is necessary but not sufficient for the development of Paget's disease of bone.J Bone Miner Res. 2014 Jun;29(6):1456-65. doi: 10.1002/jbmr.2158. J Bone Miner Res. 2014. PMID: 24339057 Free PMC article.
-
Expression of measles virus nucleocapsid protein in osteoclasts induces Paget's disease-like bone lesions in mice.J Bone Miner Res. 2006 Mar;21(3):446-55. doi: 10.1359/JBMR.051108. Epub 2005 Nov 21. J Bone Miner Res. 2006. PMID: 16491293
-
Experimental models of Paget's disease.J Bone Miner Res. 2006 Dec;21 Suppl 2:P55-7. doi: 10.1359/jbmr.06s210. J Bone Miner Res. 2006. PMID: 17229020 Review.
-
Insights into the pathogenesis of Paget's disease.Ann N Y Acad Sci. 2010 Mar;1192:176-80. doi: 10.1111/j.1749-6632.2009.05214.x. Ann N Y Acad Sci. 2010. PMID: 20392234 Review.
Cited by
-
EZH2 Supports Osteoclast Differentiation and Bone Resorption Via Epigenetic and Cytoplasmic Targets.J Bone Miner Res. 2020 Jan;35(1):181-195. doi: 10.1002/jbmr.3863. Epub 2019 Oct 23. J Bone Miner Res. 2020. PMID: 31487061 Free PMC article.
-
Pathobiology of Paget's Disease of Bone.J Bone Metab. 2014 May;21(2):85-98. doi: 10.11005/jbm.2014.21.2.85. Epub 2014 May 31. J Bone Metab. 2014. PMID: 25025000 Free PMC article. Review.
-
Measles virus nucleocapsid protein modulates the Signal Regulatory Protein-β1 (SIRPβ1) to enhance osteoclast differentiation in Paget's disease of bone.Bone Rep. 2016 Jun 14;7:26-32. doi: 10.1016/j.bonr.2016.06.002. eCollection 2017 Dec. Bone Rep. 2016. PMID: 28840181 Free PMC article.
-
Osteocytes and Paget's Disease of Bone.Curr Osteoporos Rep. 2024 Apr;22(2):266-272. doi: 10.1007/s11914-024-00863-5. Epub 2024 Mar 8. Curr Osteoporos Rep. 2024. PMID: 38457001 Free PMC article. Review.
-
TBK1 mediates critical effects of measles virus nucleocapsid protein (MVNP) on pagetic osteoclast formation.J Bone Miner Res. 2014 Jan;29(1):90-102. doi: 10.1002/jbmr.2026. J Bone Miner Res. 2014. PMID: 23794264 Free PMC article.
References
-
- Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev. 2005;208:252–66. - PubMed
-
- Helin E, Vainionpaa R, Hyypia T, Julkunen I, Matikainen S. Measles virus activates NF-kappa B and STAT transcription factors and production of IFN-alpha/beta and IL-6 in the human lung epithelial cell line A549. Virology. 2001;290:1–10. - PubMed
-
- Manchester M, Eto DS, Oldstone MB. Characterization of the inflammatory response during acute measles encephalitis in NSE-CD46 transgenic mice. J Neuroimmunol. 1999;96:207–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

