A human laboratory pilot study with baclofen in alcoholic individuals
- PMID: 23262301
- PMCID: PMC4287272
- DOI: 10.1016/j.pbb.2012.11.013
A human laboratory pilot study with baclofen in alcoholic individuals
Abstract
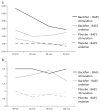
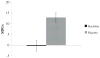
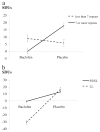
Preclinical and clinical studies show that the GABA(B) receptor agonist baclofen may represent a pharmacotherapy for alcohol dependence (AD). However, the mechanisms by which baclofen affects drinking are not well characterized; thus this pilot study investigated possible baclofen's biobehavioral mechanisms. The design was a double-blind controlled randomized human laboratory pilot study. Fourteen non-treatment seeking alcohol-dependent heavy drinking subjects received either baclofen 10mg t.i.d. or an active placebo (cyproheptadine 2mg t.i.d., to control for sedation) for a 7-day period. At day 8, participants performed an alcohol cue-reactivity (CR) followed by an alcohol self-administration (ASA). Additionally, we explored possible moderators that might guide future larger studies, i.e. anxiety, family history and onset of alcoholism, and D4 dopamine receptor (DRD4) and 5-HTTLPR polymorphisms. The main results were a significant effect of baclofen for increasing stimulation (p=.001) and sedation (p<.01). Furthermore, when drinking during the ASA and the 2 days before was analyzed as a composite variable, there was a significant effect of baclofen to reduce alcohol consumption (p<.01). As for the exploratory analyses, baclofen's effects to increase alcohol sedation and to reduce alcohol consumption were limited to those individuals with DRD4 ≥7 repeats (DRD4L). Yet, baclofen's effects on alcohol consumption were also moderated by 5-HTTLPR LL genotype. In conclusion, baclofen's ability to reduce alcohol drinking may be related to its effects on the biphasic effects of alcohol, but larger studies are needed to confirm these preliminary findings.
Published by Elsevier Inc.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
References
-
- Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II—preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. - PubMed
-
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–8. - PubMed
-
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of BACL for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–22. - PubMed
-
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F, et al. Dose–response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46:312–7. - PubMed
-
- Ameisen O. Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician. Alcohol Alcohol. 2005;40:147–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

