Pharmacokinetics, pharmacodynamics, and tolerability of GS-9851, a nucleotide analog polymerase inhibitor, following multiple ascending doses in patients with chronic hepatitis C infection
- PMID: 23263000
- PMCID: PMC3591915
- DOI: 10.1128/AAC.01263-12
Pharmacokinetics, pharmacodynamics, and tolerability of GS-9851, a nucleotide analog polymerase inhibitor, following multiple ascending doses in patients with chronic hepatitis C infection
Abstract
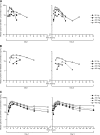
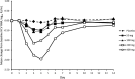
We conducted this double-blind, parallel-group, placebo-controlled, randomized, multiple-ascending-dose study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of GS-9851 (formerly PSI-7851) in treatment-naïve patients infected with hepatitis C virus (HCV) genotype 1. Thirty-two patients received active doses up to 400 mg of GS-9851 once daily for 3 days. GS-9851 and the metabolite GS-566500 (formerly PSI-352707) were rapidly cleared from the plasma, with half-life (t(1/2)) values of approximately 1 h for GS-9851 and 3 h for GS-566500. Accumulation (21%) was observed only for GS-331007 (formerly PSI-6206) after multiple dosing. GS-331007 was the primary drug-related moiety in the plasma and urine. Increases in the GS-9851, GS-566500, and GS-331007 maximum concentrations in plasma (C(max)) and area under the concentration-time curve (AUC) were less than dose proportional, particularly at the highest doses. The decline in plasma HCV RNA levels was dose dependent, and a mean maximal change from the baseline of -1.95 log(10) IU/ml was obtained for 400 mg GS-9851, compared with -0.090 log(10) IU/ml for the placebo. Most patients had a decrease in HCV RNA of ≥1.0 log(10) IU/ml after 3 days' dosing with 400 mg GS-9851. No virologic resistance was observed. GS-9851 was generally well tolerated, with no notable differences in adverse event frequency across doses. The pharmacokinetic profile observed in this study was similar to that seen in a single-ascending-dose study in healthy subjects.
Figures
References
-
- Birerdinc A, Younossi ZM. 2010. Emerging therapies for hepatitis C virus. Expert Opin. Emerg. Drugs 15:535–544 - PubMed
-
- Lauer GM, Walker BD. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41–52 - PubMed
-
- Ferenci P. 2011. Safety and efficacy of treatment for chronic hepatitis C with a focus on pegylated interferons: the backbone of therapy today and in the future. Expert Opin. Drug Saf. 10:529–544 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources