Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival
- PMID: 23263183
- PMCID: PMC4000705
- DOI: 10.1038/nature11745
Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival
Abstract
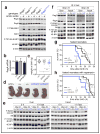
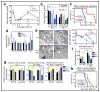
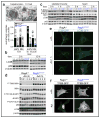
The mechanistic target of rapamycin complex 1 (mTORC1) pathway regulates organismal growth in response to many environmental cues, including nutrients and growth factors. Cell-based studies showed that mTORC1 senses amino acids through the RagA-D family of GTPases (also known as RRAGA, B, C and D), but their importance in mammalian physiology is unknown. Here we generate knock-in mice that express a constitutively active form of RagA (RagA(GTP)) from its endogenous promoter. RagA(GTP/GTP) mice develop normally, but fail to survive postnatal day 1. When delivered by Caesarean section, fasted RagA(GTP/GTP) neonates die almost twice as rapidly as wild-type littermates. Within an hour of birth, wild-type neonates strongly inhibit mTORC1, which coincides with profound hypoglycaemia and a decrease in plasma amino-acid concentrations. In contrast, mTORC1 inhibition does not occur in RagA(GTP/GTP) neonates, despite identical reductions in blood nutrient amounts. With prolonged fasting, wild-type neonates recover their plasma glucose concentrations, but RagA(GTP/GTP) mice remain hypoglycaemic until death, despite using glycogen at a faster rate. The glucose homeostasis defect correlates with the inability of fasted RagA(GTP/GTP) neonates to trigger autophagy and produce amino acids for de novo glucose production. Because profound hypoglycaemia does not inhibit mTORC1 in RagA(GTP/GTP) neonates, we considered the possibility that the Rag pathway signals glucose as well as amino-acid sufficiency to mTORC1. Indeed, mTORC1 is resistant to glucose deprivation in RagA(GTP/GTP) fibroblasts, and glucose, like amino acids, controls its recruitment to the lysosomal surface, the site of mTORC1 activation. Thus, the Rag GTPases signal glucose and amino-acid concentrations to mTORC1, and have an unexpectedly key role in neonates in autophagy induction and thus nutrient homeostasis and viability.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

