Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion
- PMID: 23263278
- PMCID: PMC4577246
- DOI: 10.1038/ncb2663
Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion
Abstract
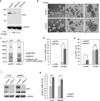
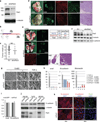
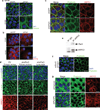
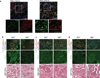
The mechanisms by which tumour cells metastasize and the role that cell polarity proteins play in this process are not well understood. We report that partitioning defective protein 3 (Par3) is dysregulated in metastasis in human breast cancer, and is associated with a higher tumour grade and ErbB2-positive status. Downregulation of Par3 cooperated with ErbB2 to induce cell invasion and metastasis in vivo. Interestingly, the metastatic behaviour was not associated with an overt mesenchymal phenotype. However, loss of Par3 inhibited E-cadherin junction stability, disrupted membrane and actin dynamics at cell-cell junctions and decreased cell-cell cohesion in a manner dependent on the Tiam1/Rac-GTP pathway. Inhibition of this pathway restored E-cadherin junction stability and blocked invasive behaviour of cells lacking Par3, suggesting that loss of Par3 promotes metastatic behaviour of ErbB2-induced tumour epithelial cells by decreasing cell-cell cohesion.
Figures




References
-
- Gomes JE, Bowerman B. Caenorhabditis elegans par genes. Curr Biol. 2002;12:R444. - PubMed
-
- Kirby C, Kusch M, Kemphues K. Mutations in the par genes of Caenorhabditis elegans affect cytoplasmic reorganization during the first cell cycle. Dev Biol. 1990;142:203–215. - PubMed
-
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. - PubMed
-
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

