An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium
- PMID: 23263281
- PMCID: PMC3701152
- DOI: 10.1038/ncb2646
An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium
Abstract
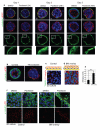
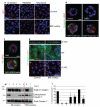
The extracellular matrix has a crucial role in determining the spatial orientation of epithelial polarity and the formation of lumens in glandular tissues; however, the underlying mechanisms remain elusive. By using Cre–Lox deletion we show that β1 integrins are required for normal mammary gland morphogenesis and lumen formation, both in vivo and in a three-dimensional primary culture model in which epithelial cells directly contact a basement membrane. Downstream of basement membrane β1 integrins, Rac1 is not involved; however, ILK is needed to polarize microtubule plus ends at the basolateral membrane and disrupting each of these components prevents lumen formation. The integrin–microtubule axis is necessary for the endocytic removal of apical proteins from the basement-membrane–cell interface and for internal Golgi positioning. We propose that this integrin signalling network controls the delivery of apical components to the correct surface and thereby governs the orientation of polarity and development of lumens.
Figures
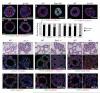

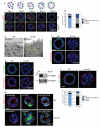
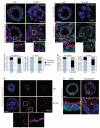
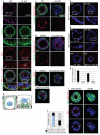

References
-
- Carmosino M, Valenti G, Caplan M, Svelto M. Polarized traffic towards the cell surface: how to find the route. Biol Cell. 102:75–91. - PubMed
-
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. - PubMed
-
- Musch A. Microtubule organization and function in epithelial cells. Traffic. 2004;5:1–9. - PubMed
-
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. - PubMed
-
- Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J Cell Sci. 1994;107(Pt 3):561–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

