Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-2 study
- PMID: 23263288
- PMCID: PMC3556255
- DOI: 10.1634/theoncologist.2012-0263
Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-2 study
Abstract
Introduction: The incidence of colorectal neuroendocrine tumors (NETs) is increasing, and patients with this disease have particularly poor prognoses. Treatment options are limited, and survival times have not improved in the past decade.
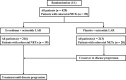
Methods: A post hoc analysis of the efficacy and tolerability of everolimus plus octreotide long-acting repeatable (LAR) was conducted in patients with colorectal NETs enrolled in the phase III RAD001 in Advanced Neuroendocrine Tumors, Second Trial (RADIANT-2) study. The primary endpoint (progression-free survival [PFS]), secondary endpoints (including objective response rate), and safety were assessed.
Results: Patients with colorectal NETs receiving everolimus plus octreotide LAR had a significantly longer median PFS (29.9 months; n = 19) than did those receiving placebo plus octreotide LAR (6.6 months; n = 20). Everolimus plus octreotide LAR treatment also significantly reduced the risk for disease progression (hazard ratio: 0.34; 95% confidence interval: 0.13-0.89; p = .011). Although no objective responses were observed, tumor shrinkage was more frequently noted in the everolimus plus octreotide LAR arm than in the placebo plus octreotide LAR arm (67% vs. 37%, respectively). The combination of everolimus plus octreotide LAR was generally well tolerated by patients with colorectal NETs; rash and stomatitis were the most commonly reported adverse events.
Conclusions: Everolimus plus octreotide LAR treatment had significant benefits and improved outcomes for patients with advanced colorectal NETs compared with placebo plus octreotide LAR treatment. Results of this exploratory analysis are consistent with those reported from the RADIANT-2 primary analysis. These findings support additional investigations of everolimus plus octreotide LAR in patients with colorectal NETs.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures



References
-
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. - PubMed
-
- Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(Suppl 1):3–7. - PubMed
-
- Kang H, O'Connell JB, Leonardi MJ, et al. Rare tumors of the colon and rectum: A national review. Int J Colorectal Dis. 2007;22:183–189. - PubMed
-
- Anthony LB, Strosberg JR, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (NETs): Well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767–774. - PubMed
-
- Schimmack S, Svejda B, Lawrence B, et al. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch Surg. 2011;396:273–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

