A subpopulation of nociceptors specifically linked to itch
- PMID: 23263443
- PMCID: PMC3557753
- DOI: 10.1038/nn.3289
A subpopulation of nociceptors specifically linked to itch
Abstract
Itch-specific neurons have been sought for decades. The existence of such neurons has been doubted recently as a result of the observation that itch-mediating neurons also respond to painful stimuli. We genetically labeled and manipulated MrgprA3(+) neurons in the dorsal root ganglion (DRG) and found that they exclusively innervated the epidermis of the skin and responded to multiple pruritogens. Ablation of MrgprA3(+) neurons led to substantial reductions in scratching evoked by multiple pruritogens and occurring spontaneously under chronic itch conditions, whereas pain sensitivity remained intact. Notably, mice in which TRPV1 was exclusively expressed in MrgprA3(+) neurons exhibited itch, but not pain, behavior in response to capsaicin. Although MrgprA3(+) neurons were sensitive to noxious heat, activation of TRPV1 in these neurons by noxious heat did not alter pain behavior. These data suggest that MrgprA3 defines a specific subpopulation of DRG neurons mediating itch. Our study opens new avenues for studying itch and developing anti-pruritic therapies.
Figures
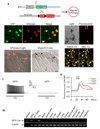
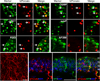
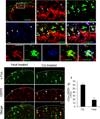


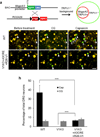
Comment in
-
Scratching an itch.Nat Neurosci. 2013 Feb;16(2):117-8. doi: 10.1038/nn.3316. Nat Neurosci. 2013. PMID: 23354383 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- NS014624/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 EY017203/EY/NEI NIH HHS/United States
- NS070814/NS/NINDS NIH HHS/United States
- GM087369/GM/NIGMS NIH HHS/United States
- NS047399/NS/NINDS NIH HHS/United States
- R01 NS014624/NS/NINDS NIH HHS/United States
- R37 NS054791/NS/NINDS NIH HHS/United States
- R01 GM087369/GM/NIGMS NIH HHS/United States
- P01 NS047399/NS/NINDS NIH HHS/United States
- NS054791/NS/NINDS NIH HHS/United States
- R01 NS070814/NS/NINDS NIH HHS/United States
- R01 NS054791/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

