Potential impact of microbial activity on the oxidant capacity and organic carbon budget in clouds
- PMID: 23263871
- PMCID: PMC3545818
- DOI: 10.1073/pnas.1205743110
Potential impact of microbial activity on the oxidant capacity and organic carbon budget in clouds
Abstract
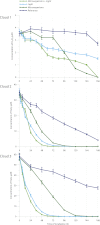
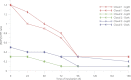
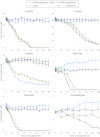
Within cloud water, microorganisms are metabolically active and, thus, are expected to contribute to the atmospheric chemistry. This article investigates the interactions between microorganisms and the reactive oxygenated species that are present in cloud water because these chemical compounds drive the oxidant capacity of the cloud system. Real cloud water samples with contrasting features (marine, continental, and urban) were taken from the puy de Dôme mountain (France). The samples exhibited a high microbial biodiversity and complex chemical composition. The media were incubated in the dark and subjected to UV radiation in specifically designed photo-bioreactors. The concentrations of H(2)O(2), organic compounds, and the ATP/ADP ratio were monitored during the incubation period. The microorganisms remained metabolically active in the presence of ()OH radicals that were photo-produced from H(2)O(2). This oxidant and major carbon compounds (formaldehyde and carboxylic acids) were biodegraded by the endogenous microflora. This work suggests that microorganisms could play a double role in atmospheric chemistry; first, they could directly metabolize organic carbon species, and second, they could reduce the available source of radicals through their oxidative metabolism. Consequently, molecules such as H(2)O(2) would no longer be available for photochemical or other chemical reactions, which would decrease the cloud oxidant capacity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lim YB, Tan Y, Perri MJ, Seitzinger SP, Turpin BJ. Aqueous chemistry and its role in secondary organic aerosol (SOA) formation. Atmos Chem Phys. 2010;10(21):10521–10539.
-
- Ervens B, Turpin BJ, Weber RJ. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos Chem Phys. 2011;11(21):11069–11102.
-
- Solomon S, et al., editors. Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK, and New York, NY: Cambridge University Press; 2007. Contribution of Working Group I.
-
- Herrmann H, Hoffmann D, Schaefer T, Bräuer P, Tilgner A. Tropospheric aqueous-phase free-radical chemistry: Radical sources, spectra, reaction kinetics and prediction tools. ChemPhysChem. 2010;11(18):3796–3822. - PubMed
-
- Deguillaume L, et al. Transition metals in atmospheric liquid phases: Sources, reactivity, and sensitive parameters. Chem Rev. 2005;105(9):3388–3431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

