Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance
- PMID: 23264516
- PMCID: PMC3542046
- DOI: 10.1093/jxb/ers347
Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance
Abstract
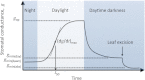
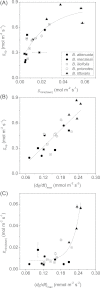
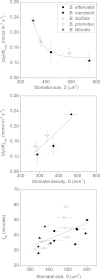
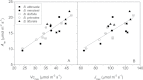
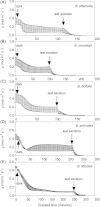
Maximum and minimum stomatal conductance, as well as stomatal size and rate of response, are known to vary widely across plant species, but the functional relationship between these static and dynamic stomatal properties is unknown. The objective of this study was to test three hypotheses: (i) operating stomatal conductance under standard conditions (g (op)) correlates with minimum stomatal conductance prior to morning light [g (min(dawn))]; (ii) stomatal size (S) is negatively correlated with g (op) and the maximum rate of stomatal opening in response to light, (dg/dt)(max); and (iii) g (op) correlates negatively with instantaneous water-use efficiency (WUE) despite positive correlations with maximum rate of carboxylation (Vc (max)) and light-saturated rate of electron transport (J (max)). Using five closely related species of the genus Banksia, the above variables were measured, and it was found that all three hypotheses were supported by the results. Overall, this indicates that leaves built for higher rates of gas exchange have smaller stomata and faster dynamic characteristics. With the aid of a stomatal control model, it is demonstrated that higher g (op) can potentially expose plants to larger tissue water potential gradients, and that faster stomatal response times can help offset this risk.
Figures




References
-
- Aasama K, Sõber A, Rahi M. 2001. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Australian Journal of Plant Physiology 28, 765–774
-
- Anderson JE, Williams J, Kriedemann PE, Austin MP, Farquhar GD. 1996. Correlations between carbon isotope discrimination and climate of native habitats for diverse eucalypt taxa growing in a common garden. Australian Journal of Plant Physiology 23, 311–320
-
- Assmann SM, Grantz DA. 1990. Stomatal response to humidity in sugarcane and soybean: effect of vapour pressure difference on the kinetics of the blue light response. Plant, Cell and Environment 13, 163–169
-
- Barbour MM, Buckley TN. 2007. The stomatal response to evaporative demand persists at night in Ricinus communis plants with high nocturnal conductance. Plant, Cell and Environment 30, 711–721 - PubMed
-
- Barbour MM, Cernusak LA, Whitehead D, Griffin KL, Turnbull MH, Tissue DT, Farquhar GD. 2005. Nocturnal stomatal conductance and implications for modelling δ18O of leaf-respired CO2 in temperate tree species. Functional Plant Biology 32, 1107–1121 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

