A patient-derived stem cell model of hereditary spastic paraplegia with SPAST mutations
- PMID: 23264559
- PMCID: PMC3597030
- DOI: 10.1242/dmm.010884
A patient-derived stem cell model of hereditary spastic paraplegia with SPAST mutations
Erratum in
-
A patient-derived stem cell model of hereditary spastic paraplegia with SPAST mutations.Dis Model Mech. 2015 Oct 1;8(10):1339. doi: 10.1242/dmm.023002. Dis Model Mech. 2015. PMID: 26438696 Free PMC article. No abstract available.
Abstract
Hereditary spastic paraplegia (HSP) leads to progressive gait disturbances with lower limb muscle weakness and spasticity. Mutations in SPAST are a major cause of adult-onset, autosomal-dominant HSP. Spastin, the protein encoded by SPAST, is a microtubule-severing protein that is enriched in the distal axon of corticospinal motor neurons, which degenerate in HSP patients. Animal and cell models have identified functions of spastin and mutated spastin but these models lack the gene dosage, mutation variability and genetic background that characterize patients with the disease. In this study, this genetic variability is encompassed by comparing neural progenitor cells derived from biopsies of the olfactory mucosa from healthy controls with similar cells from HSP patients with SPAST mutations, in order to identify cell functions altered in HSP. Patient-derived cells were similar to control-derived cells in proliferation and multiple metabolic functions but had major dysregulation of gene expression, with 57% of all mRNA transcripts affected, including many associated with microtubule dynamics. Compared to control cells, patient-derived cells had 50% spastin, 50% acetylated α-tubulin and 150% stathmin, a microtubule-destabilizing enzyme. Patient-derived cells were smaller than control cells. They had altered intracellular distributions of peroxisomes and mitochondria and they had slower moving peroxisomes. These results suggest that patient-derived cells might compensate for reduced spastin, but their increased stathmin expression reduced stabilized microtubules and altered organelle trafficking. Sub-nanomolar concentrations of the microtubule-binding drugs, paclitaxel and vinblastine, increased acetylated α-tubulin levels in patient cells to control levels, indicating the utility of this cell model for screening other candidate compounds for drug therapies.
Figures



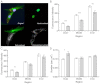
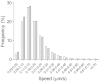

References
-
- Cassimeris L. (2002). The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 14, 18-24 - PubMed
-
- Charvin D., Cifuentes-Diaz C., Fonknechten N., Joshi V., Hazan J., Melki J., Betuing S. (2003). Mutations of SPG4 are responsible for a loss of function of spastin, an abundant neuronal protein localized in the nucleus. Hum. Mol. Genet. 12, 71-78 - PubMed
-
- Claudiani P., Riano E., Errico A., Andolfi G., Rugarli E. I. (2005). Spastin subcellular localization is regulated through usage of different translation start sites and active export from the nucleus. Exp. Cell Res. 309, 358-369 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

