A new and clinically relevant murine model of solid-organ transplant aspergillosis
- PMID: 23264562
- PMCID: PMC3634648
- DOI: 10.1242/dmm.010330
A new and clinically relevant murine model of solid-organ transplant aspergillosis
Abstract
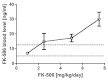
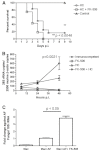
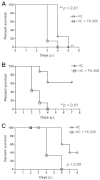
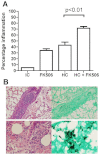
Invasive fungal infections (IFIs) are a major cause of death in organ transplant patients. The murine hydrocortisone-mediated immunosuppression model of pulmonary aspergillosis is commonly used to characterise IFIs in these patients. However, this model does not take into account the effects of calcineurin inhibitors on transplant immunity to IFIs or the fungal calcineurin pathway, which is required for both virulence and antifungal drug resistance. To address these two issues, a new and clinically relevant transplant immunosuppression model of tacrolimus (FK506) and hydrocortisone-associated pulmonary aspergillosis was developed. We first characterised IFIs in 406 patients with a lung transplant. This showed that all of the patients with pulmonary aspergillosis were immunosuppressed with calcineurin inhibitors and steroids. Murine pharmacokinetic studies demonstrated that an ideal dose of 1 mg/kg/day of FK506 intraperitoneally produced blood trough levels in the human therapeutic range (5-12 ng/ml). There was increased mortality from pulmonary aspergillosis in a transplant-relevant immunosuppression model using both FK506 and hydrocortisone as compared with immunosuppression using hydrocortisone only. Lung histopathology showed neutrophil invasion and tracheobronchitis that was associated with reduced lung tumour necrosis factor-α (TNFα), JE (homologue of human MCP-1) and KC (homologue of human IL-8) at 24 hours, but increased lung TNFα, JE and KC at 48 hours when fungal burden was high. Furthermore, FK506 directly impaired fungal killing in alveolar macrophages in vitro, with FK506-mediated inhibition of the radial growth of Aspergillus fumigatus in vitro occurring at the low concentration of 5 ng/ml. Taken together, these findings show that the immunosuppressive activity of FK506 outweighs its antifungal activity in vivo. These observations demonstrate that FK506 impairs innate immune responses and leads to an incremental increase in susceptibility to IFIs when it is combined with steroids. This new and clinically relevant mouse model of invasive aspergillosis is a valuable addition to the further study of both fungal immunity and antifungal therapy in organ transplantation.
Figures

Similar articles
-
Three-Dimensional Light Sheet Fluorescence Microscopy of Lungs To Dissect Local Host Immune-Aspergillus fumigatus Interactions.mBio. 2020 Feb 4;11(1):e02752-19. doi: 10.1128/mBio.02752-19. mBio. 2020. PMID: 32019790 Free PMC article.
-
Neuroimmune Responses in a New Experimental Animal Model of Cerebral Aspergillosis.mBio. 2022 Oct 26;13(5):e0225422. doi: 10.1128/mbio.02254-22. Epub 2022 Aug 30. mBio. 2022. PMID: 36040029 Free PMC article.
-
In Vitro and In Vivo Assessment of FK506 Analogs as Novel Antifungal Drug Candidates.Antimicrob Agents Chemother. 2018 Oct 24;62(11):e01627-18. doi: 10.1128/AAC.01627-18. Print 2018 Nov. Antimicrob Agents Chemother. 2018. PMID: 30181374 Free PMC article.
-
Invasive pulmonary Aspergillosis in organ transplants--Focus on lung transplants.Respir Investig. 2016 Mar;54(2):76-84. doi: 10.1016/j.resinv.2015.08.005. Epub 2015 Nov 10. Respir Investig. 2016. PMID: 26879476 Review.
-
The clinical spectrum of pulmonary aspergillosis.Thorax. 2015 Mar;70(3):270-7. doi: 10.1136/thoraxjnl-2014-206291. Epub 2014 Oct 29. Thorax. 2015. PMID: 25354514 Review.
Cited by
-
Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus.EMBO Mol Med. 2015 Mar;7(3):240-58. doi: 10.15252/emmm.201404556. EMBO Mol Med. 2015. PMID: 25637383 Free PMC article.
-
FK506 impairs neutrophil migration that results in increased polymicrobial sepsis susceptibility.Inflamm Res. 2023 Feb;72(2):203-215. doi: 10.1007/s00011-022-01669-w. Epub 2022 Nov 19. Inflamm Res. 2023. PMID: 36401631
-
Vaccine-Induced Protection in Two Murine Models of Invasive Pulmonary Aspergillosis.Front Immunol. 2021 May 18;12:670578. doi: 10.3389/fimmu.2021.670578. eCollection 2021. Front Immunol. 2021. PMID: 34084170 Free PMC article.
-
Commentary: Experimental Mouse Models of Invasive Candidiasis Caused by Candida auris and Other Medically Important Candida Species.J Cell Immunol. 2022;4(1):29-33. doi: 10.33696/immunology.4.130. J Cell Immunol. 2022. PMID: 35847345 Free PMC article.
-
Immunosuppression Affects Neutrophil Functions: Does Calcineurin-NFAT Signaling Matter?Front Immunol. 2021 Nov 2;12:770515. doi: 10.3389/fimmu.2021.770515. eCollection 2021. Front Immunol. 2021. PMID: 34795676 Free PMC article. Review.
References
-
- De Pauw B., Walsh T. J., Donnelly J. P., Stevens D. A., Edwards J. E., Calandra T., Pappas P. G., Maertens J., Lortholary O., Kauffman C. A., et al. (2008). Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821 - PMC - PubMed
-
- Fishman J. A. (2007). Infection in solid-organ transplant recipients. N. Engl. J. Med. 357, 2601–2614 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

