Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling
- PMID: 23264597
- PMCID: PMC4968370
- DOI: 10.1182/blood-2012-08-452607
Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling
Abstract
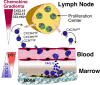
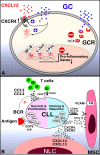
Chronic lymphocytic leukemia (CLL) cells proliferate in pseudofollicles within the lymphatic tissues, where signals from the microenvironment and BCR signaling drive the expansion of the CLL clone. Mobilization of tissue-resident cells into the blood removes CLL cells from this nurturing milieu and sensitizes them to cytotoxic drugs. This concept recently gained momentum after the clinical activity of kinase inhibitors that target BCR signaling (spleen tyrosine kinase, Bruton tyrosine kinase, PI3Kδ inhibitors) was established. Besides antiproliferative activity, these drugs cause CLL cell redistribution with rapid lymph node shrinkage, along with a transient surge in lymphocytosis, before inducing objective remissions. Inactivation of critical CLL homing mechanism (chemokine receptors, adhesion molecules), thwarting tissue retention and recirculation into the tissues, appears to be the basis for this striking clinical activity. This effect of BCR-signaling inhibitors resembles redistribution of CLL cells after glucocorticoids, described as early as in the 1940s. As such, we are witnessing a renaissance of the concept of leukemia cell redistribution in modern CLL therapy. Here, we review the molecular basis of CLL cell trafficking, homing, and redistribution and similarities between old and new drugs affecting these processes. In addition, we outline how these discoveries are changing our understanding of CLL biology and therapy.
Figures

References
-
- Stein H, Bonk A, Tolksdorf G, Lennert K, Rodt H, Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980;28(8):746–760. - PubMed
-
- Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 2002;99(3):1030–1037. - PubMed
-
- Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110(9):3316–3325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

