Regulation of TLR2-mediated tolerance and cross-tolerance through IRAK4 modulation by miR-132 and miR-212
- PMID: 23264652
- PMCID: PMC3552145
- DOI: 10.4049/jimmunol.1103060
Regulation of TLR2-mediated tolerance and cross-tolerance through IRAK4 modulation by miR-132 and miR-212
Abstract
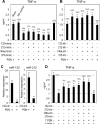
Innate immune response is the first defense against pathogens via recognition by various conserved pattern recognition receptors, such as TLRs, to initiate a rapid and strong cytokine alarm. TLR signaling-mediated cytokine production must be properly regulated to prevent pathological conditions deriving from overproduction of cytokines. In this study, the role of specific microRNAs in TLR-signaling pathway was investigated to reveal the cross-interaction and -regulation in the MyD88 pathway. In peptidoglycan (PGN)/TLR2-stimulated THP-1 monocytes, PBMCs, and primary macrophages showed rapid and dramatic miR-132 and miR-212 (miR-132/-212) upregulation. This newly identified response appeared earlier in time than the characteristic miR-146a response in LPS-TLR4 stimulation. The rapid induction of miR-132/-212 was transcription factor CREB dependent, and the sustained expression of miR-132/-212 was responsible for inducing tolerance to subsequent PGN challenge. Cross-tolerance was observed by TLR5 ligand flagellin and heat-killed or live bacteria resulting from miR-132/-212 upregulation. Mechanistically, IRAK4 was identified and validated as a target of miR-132/-212 by luciferase reporter assay and seed-sequence mutagenesis of the reporter. Transfection of miR-132 or miR-212 alone mimicked PGN tolerance in monocytes, whereas transfected specific miRNA inhibitors tampered the tolerance effect. During bacterial infection, PGN-mediated TLR2 signaling induces miR-132/-212 to downregulate IRAK4, an early component in the MyD88-dependent pathway, whereas LPS/TLR4-induced miR-146a downregulates downstream components of the same MyD88-dependent pathway. The identification of miR-132/-212 and miR-146a together to prevent damaging consequences from the overproduction of proinflammatory cytokines by targeting a common signaling pathway is significant and will provide insights into future design and development of therapeutics.
Figures





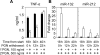
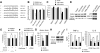

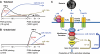
References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat.Immunol. 2004;5:975–979. - PubMed
-
- de Vos AF, Pater JM, van den Pangaart PS, de Kruif MD, van 't Veer C, van der Poll T. In vivo lipopolysaccharide exposure of human blood leukocytes induces cross-tolerance to multiple TLR ligands. J. Immunol. 2009;183:533–542. - PubMed
-
- del Fresno C, Garcia-Rio F, Gomez-Pina V, Soares-Schanoski A, Fernandez-Ruiz I, Jurado T, Kajiji T, Shu C, Marin E, Gutierrez del Arroyo A, Prados C, Arnalich F, Fuentes-Prior P, Biswas SK, Lopez-Collazo E. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J. Immunol. 2009;182:6494–6507. - PubMed
-
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

