Surrogate endpoint analysis: an exercise in extrapolation
- PMID: 23264679
- PMCID: PMC3611854
- DOI: 10.1093/jnci/djs527
Surrogate endpoint analysis: an exercise in extrapolation
Abstract
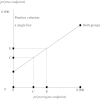
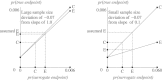
Surrogate endpoints offer the hope of smaller or shorter cancer trials. It is, however, important to realize they come at the cost of an unverifiable extrapolation that could lead to misleading conclusions. With cancer prevention, the focus is on hypothesis testing in small surrogate endpoint trials before deciding whether to proceed to a large prevention trial. However, it is not generally appreciated that a small surrogate endpoint trial is highly sensitive to a deviation from the key Prentice criterion needed for the hypothesis-testing extrapolation. With cancer treatment, the focus is on estimation using historical trials with both surrogate and true endpoints to predict treatment effect based on the surrogate endpoint in a new trial. Successively leaving out one historical trial and computing the predicted treatment effect in the left-out trial yields a standard error multiplier that summarizes the increased uncertainty in estimation extrapolation. If this increased uncertainty is acceptable, three additional extrapolation issues (biological mechanism, treatment following observation of the surrogate endpoint, and side effects following observation of the surrogate endpoint) need to be considered. In summary, when using surrogate endpoint analyses, an appreciation of the problems of extrapolation is crucial.
Figures
References
-
- Torri V, Simon R, Russek-Cohen E, Midthune D, Friedman M. Statistical model to determine the relationship of response and survival in patients with advanced ovarian cancer treated with chemotherapy. J Natl Cancer Inst. 1992; 84(6):407–414 - PubMed
-
- Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic disease. Stat Med. 1992; 11(2)167–178 - PubMed
-
- Daniels MJ, Hughes MD. Meta-analysis for the evaluation of potential surrogate markers. Stat Med. 1997; 16(17):1965–1982 - PubMed
-
- Gail MH, Pfeiffer R, Houwelingen HC, Carroll RJ. On meta-analytic assessment of surrogate outcomes. Biostatistics. 2000; 1(3):231–246 - PubMed
-
- Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000; 1(1):49–67 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

