Iron metabolism in aerobes: managing ferric iron hydrolysis and ferrous iron autoxidation
- PMID: 23264695
- PMCID: PMC3524981
- DOI: 10.1016/j.ccr.2012.06.030
Iron metabolism in aerobes: managing ferric iron hydrolysis and ferrous iron autoxidation
Abstract
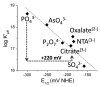
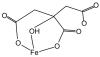
Aerobes and anaerobes alike express a plethora of essential iron enzymes; in the resting state, the iron atom(s) in these proteins are in the ferrous state. For aerobes, ferric iron is the predominant environmental valence form which, given ferric iron's aqueous chemistry, occurs as 'rust', insoluble, bio-inert polymeric ferric oxide that results from the hydrolysis of [Fe(H(2)O)(6)](3+). Mobilizing this iron requires bio-ferrireduction which in turn requires managing the rapid autoxidation of the resulting Fe(II) which occurs at pH > 6. This review examines the aqueous redox chemistry of iron and the mechanisms evolved in aerobes to suppress the 'rusting out' of Fe(III) and the ROS-generating autoxidation of Fe(II) so as to make this metal ion available as the most ubiquitous prosthetic group in metallobiology.
Figures

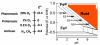



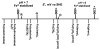




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
