Activation of PAD4 in NET formation
- PMID: 23264775
- PMCID: PMC3525017
- DOI: 10.3389/fimmu.2012.00360
Activation of PAD4 in NET formation
Abstract
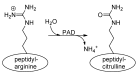
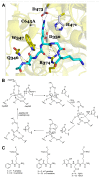
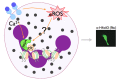
Peptidylarginine deiminases, or PADs, convert arginine residues to the non-ribosomally encoded amino acid citrulline in a variety of protein substrates. PAD4 is expressed in granulocytes and is essential for the formation of neutrophil extracellular traps (NETs) via PAD4-mediated histone citrullination. Citrullination of histones is thought to promote NET formation by inducing chromatin decondensation and facilitating the expulsion of chromosomal DNA that is coated with antimicrobial molecules. Numerous stimuli have been reported to lead to PAD4 activation and NET formation. However, how this signaling process proceeds and how PAD4 becomes activated in cells is largely unknown. Herein, we describe the various stimuli and signaling pathways that have been implicated in PAD4 activation and NET formation, including the role of reactive oxygen species generation. To provide a foundation for the above discussion, we first describe PAD4 structure and function, and how these studies led to the development of PAD-specific inhibitors. A comprehensive survey of the receptors and signaling pathways that regulate PAD4 activation will be important for our understanding of innate immunity, and the identification of signaling intermediates in PAD4 activation may also lead to the generation of pharmaceuticals to target NET-related pathogenesis.
Keywords: NET; PAD4; citrullination; deimination; neutrophil.
Figures
Similar articles
-
Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation.J Cell Biol. 2009 Jan 26;184(2):205-13. doi: 10.1083/jcb.200806072. Epub 2009 Jan 19. J Cell Biol. 2009. PMID: 19153223 Free PMC article.
-
ROLE OF PEPTIDYLARGININE DEIMINASE AND NEUTROPHIL EXTRACELLULAR TRAPS IN INJURIES: FUTURE NOVEL DIAGNOSTICS AND THERAPEUTIC TARGETS.Shock. 2023 Feb 1;59(2):247-255. doi: 10.1097/SHK.0000000000002052. Epub 2023 Jan 5. Shock. 2023. PMID: 36597759 Free PMC article. Review.
-
PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures.Front Immunol. 2012 Oct 4;3:307. doi: 10.3389/fimmu.2012.00307. eCollection 2012. Front Immunol. 2012. PMID: 23060885 Free PMC article.
-
Candida albicans-Induced NETosis Is Independent of Peptidylarginine Deiminase 4.Front Immunol. 2018 Jul 9;9:1573. doi: 10.3389/fimmu.2018.01573. eCollection 2018. Front Immunol. 2018. PMID: 30038623 Free PMC article.
-
PAD4 takes charge during neutrophil activation: Impact of PAD4 mediated NET formation on immune-mediated disease.J Thromb Haemost. 2021 Jul;19(7):1607-1617. doi: 10.1111/jth.15313. Epub 2021 May 12. J Thromb Haemost. 2021. PMID: 33773016 Free PMC article. Review.
Cited by
-
Mechanisms regulating neutrophil survival and cell death.Semin Immunopathol. 2013 Jul;35(4):423-37. doi: 10.1007/s00281-013-0364-x. Epub 2013 Feb 1. Semin Immunopathol. 2013. PMID: 23370701 Review.
-
Neutrophil Extracellular Traps in Digestive Cancers: Warrior or Accomplice.Front Oncol. 2021 Nov 19;11:766636. doi: 10.3389/fonc.2021.766636. eCollection 2021. Front Oncol. 2021. PMID: 34868992 Free PMC article. Review.
-
NETosis and the Immune System in COVID-19: Mechanisms and Potential Treatments.Front Pharmacol. 2021 Aug 5;12:708302. doi: 10.3389/fphar.2021.708302. eCollection 2021. Front Pharmacol. 2021. PMID: 34421600 Free PMC article. Review.
-
Lupus and proliferative nephritis are PAD4 independent in murine models.JCI Insight. 2017 May 18;2(10):e92926. doi: 10.1172/jci.insight.92926. eCollection 2017 May 18. JCI Insight. 2017. PMID: 28515361 Free PMC article.
-
Acute Kidney Injury Induced Lupus Exacerbation Through the Enhanced Neutrophil Extracellular Traps (and Apoptosis) in Fcgr2b Deficient Lupus Mice With Renal Ischemia Reperfusion Injury.Front Immunol. 2021 Jun 24;12:669162. doi: 10.3389/fimmu.2021.669162. eCollection 2021. Front Immunol. 2021. PMID: 34248948 Free PMC article.
References
-
- Amulic B., Cazalet C., Hayes G. L., Metzler K. D., Zychlinsky A. (2012). Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30 459–489 - PubMed
-
- Arita K., Hashimoto H., Shimizu T., Nakashima K., Yamada M., Sato M. (2004). Structural basis for Ca2+-induced activation of human PAD4. Nat. Struct. Mol. Biol. 11 777–783 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

