Biocompatible Gold Nanorod Conjugates for Preclinical Biomedical Research
- PMID: 23264890
- PMCID: PMC3526006
- DOI: 10.4172/2157-7439.S2-001
Biocompatible Gold Nanorod Conjugates for Preclinical Biomedical Research
Abstract
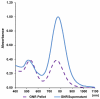
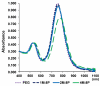
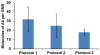
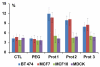
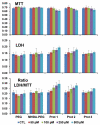
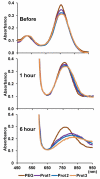
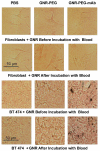
Gold nanorods with a peak absorption wavelength of 760 nm were prepared using a seed-mediated method. A novel protocol has been developed to replace hexadecyltrimethylammonium bromide on the surface of the nanorods with 16-mercaptohexadecanoic acid and metoxy-poly(ethylene glycol)-thiol, and the monoclonal antibody HER2. The physical chemistry properties of the conjugates were monitored through optical and zeta-potential measurements to confirm surface chemistry changes. The efficiency of the modifications was quantified through measurement of the average number of antibodies per gold nanorod. The conjugates were investigated for different cells lines: BT-474, MCF7, MCF10, MDCK, and fibroblast. The results show successful cell accumulation of the gold nanorod HER2 conjugates in cells with HER2 overexpression. Incubation of the complexes in heparinized mouse blood demonstrated the low aggregation of the metallic particles through stability of the spectral properties, as verified by UV/VIS spectrometry. Cytotoxicity analysis with LDH release and MTT assay confirms strong targeting and retention of functional activity of the antibody after their conjugation with gold nanorods. Silver staining confirms efficient specific binding to BT-474 cells even in cases where the nanorod complexes were incubated in heparinized mouse blood. This is confirmed through in vivo studies where, following intravenous injection of gold nanorod complexes, silver staining reveals noticeably higher rates of specific binding in mouse tumors than in healthy liver.The conjugates are reproducible, have strong molecular targeting capabilities, have long term stability in vivo and can be used in pre-clinical applications. The conjugates can also be used for molecular and optoacoustic imaging, quantitative sensing of biological substrates, and photothermal therapy.
Figures


Similar articles
-
Melanin nanoparticles as a novel contrast agent for optoacoustic tomography.Photoacoustics. 2015 Feb 14;3(1):35-43. doi: 10.1016/j.pacs.2015.02.001. eCollection 2015 Mar. Photoacoustics. 2015. PMID: 25893172 Free PMC article.
-
Polymer-Peptide Modified Gold Nanorods to Improve Cell Conjugation and Cell labelling for Stem Cells Photoacoustic Imaging.Diagnostics (Basel). 2021 Jun 30;11(7):1196. doi: 10.3390/diagnostics11071196. Diagnostics (Basel). 2021. PMID: 34209370 Free PMC article.
-
Conjugation of antibodies to gold nanorods through Fc portion: synthesis and molecular specific imaging.Bioconjug Chem. 2013 Jun 19;24(6):878-88. doi: 10.1021/bc3004815. Epub 2013 May 14. Bioconjug Chem. 2013. PMID: 23631707 Free PMC article.
-
A Review on Cancer Therapy Based on the Photothermal Effect of Gold Nanorod.Curr Pharm Des. 2019;25(46):4836-4847. doi: 10.2174/1381612825666191216150052. Curr Pharm Des. 2019. PMID: 31840600 Review.
-
Gold Nanorods as Nanodevices for Bioimaging, Photothermal Therapeutics, and Drug Delivery.Chem Pharm Bull (Tokyo). 2017;65(7):625-628. doi: 10.1248/cpb.c17-00102. Chem Pharm Bull (Tokyo). 2017. PMID: 28674334 Review.
Cited by
-
Hairpin Oligonucleotide Can Functionalize Gold Nanorods for in Vivo Application Delivering Cytotoxic Nucleotides and Curcumin: A Comprehensive Study in Combination with Near-Infrared Laser.ACS Omega. 2020 Nov 2;5(44):28463-28474. doi: 10.1021/acsomega.0c02288. eCollection 2020 Nov 10. ACS Omega. 2020. PMID: 33195896 Free PMC article.
-
Pancreatic tumor microenvironmental acidosis and hypoxia transform gold nanorods into cell-penetrant particles for potent radiosensitization.Sci Adv. 2022 Nov 11;8(45):eabm9729. doi: 10.1126/sciadv.abm9729. Epub 2022 Nov 11. Sci Adv. 2022. PMID: 36367938 Free PMC article.
-
Melanin nanoparticles as a novel contrast agent for optoacoustic tomography.Photoacoustics. 2015 Feb 14;3(1):35-43. doi: 10.1016/j.pacs.2015.02.001. eCollection 2015 Mar. Photoacoustics. 2015. PMID: 25893172 Free PMC article.
-
Ready-to-use protein G-conjugated gold nanorods for biosensing and biomedical applications.J Nanobiotechnology. 2018 Jan 19;16(1):5. doi: 10.1186/s12951-017-0329-7. J Nanobiotechnology. 2018. PMID: 29351815 Free PMC article.
-
Photothermolysis mediated by gold nanorods modified with EGFR monoclonal antibody induces Hep-2 cells apoptosis in vitro and in vivo.Int J Nanomedicine. 2014 Apr 17;9:1931-46. doi: 10.2147/IJN.S59870. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24790435 Free PMC article.
References
-
- Liao H, Hafner JH. Gold Nanorod Bioconjugates. Chem Mater. 2005;17:4636–4641.
-
- Perez-Juste J, Pastoria-Santos I, Liz-Marzan LM, Mulvaney P. Gold nanorods: Synthesis, characterization, and applications. Coord Chem Rev. 2005;249:1870–1901.
-
- Huang X, El-Sayed IH, El-Sayed MA. Applications of Gold Nanorods for Cancer Imaging and Photothermal Therapy. Methods Mol Biol. 2010;624:343–357. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
