How do CARs work?: Early insights from recent clinical studies targeting CD19
- PMID: 23264903
- PMCID: PMC3525612
- DOI: 10.4161/onci.22524
How do CARs work?: Early insights from recent clinical studies targeting CD19
Abstract
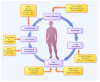
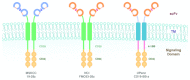
Second-generation chimeric antigen receptors (CARs) are powerful tools to redirect antigen-specific T cells independently of HLA-restriction. Recent clinical studies evaluating CD19-targeted T cells in patients with B-cell malignancies demonstrate the potency of CAR-engineered T cells. With results from 28 subjects enrolled by five centers conducting studies in patients with chronic lymphocytic leukemia (CLL) or lymphoma, some insights into the parameters that determine T-cell function and clinical outcome of CAR-based approaches are emerging. These parameters involve CAR design, T-cell production methods, conditioning chemotherapy as well as patient selection. Here, we discuss the potential relevance of these findings and in particular the interplay between the adoptive transfer of T cells and pre-transfer patient conditioning.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
