Iron(II) bromide-catalyzed intramolecular C-H bond amination [1,2]-shift tandem reactions of aryl azides
- PMID: 23265139
- PMCID: PMC3622273
- DOI: 10.1021/ja3113565
Iron(II) bromide-catalyzed intramolecular C-H bond amination [1,2]-shift tandem reactions of aryl azides
Abstract
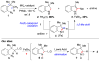
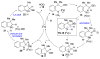
Iron(II) bromide catalyzes the transformation of ortho-substituted aryl azides into 2,3-disubstituted indoles through a tandem ethereal C-H bond amination [1,2]-shift reaction. The preference for the 1,2-shift component of the tandem reaction was established to be Me < 1° < 2° < Ph.
Figures
Similar articles
-
Intramolecular Ir(I)-catalyzed benzylic C-H bond amination of ortho-substituted aryl azides.Org Lett. 2009 Aug 20;11(16):3598-601. doi: 10.1021/ol901317j. Org Lett. 2009. PMID: 19627144 Free PMC article.
-
Iron(II) bromide-catalyzed synthesis of benzimidazoles from aryl azides.Org Lett. 2008 Aug 7;10(15):3367-70. doi: 10.1021/ol801227f. Epub 2008 Jul 3. Org Lett. 2008. PMID: 18597473
-
Intramolecular Fe(II)-catalyzed N-O or N-N bond formation from aryl azides.Org Lett. 2010 Jun 18;12(12):2884-7. doi: 10.1021/ol101040p. Org Lett. 2010. PMID: 20507088 Free PMC article.
-
Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 2-Azides, Phthalocyanines, Subphthalocyanines and Porphyrazines.Molecules. 2020 Apr 10;25(7):1745. doi: 10.3390/molecules25071745. Molecules. 2020. PMID: 32290240 Free PMC article. Review.
-
Azides in the Synthesis of Various Heterocycles.Molecules. 2022 Jun 9;27(12):3716. doi: 10.3390/molecules27123716. Molecules. 2022. PMID: 35744839 Free PMC article. Review.
Cited by
-
Silver Catalyzed Site-Selective C(sp3)-H Bond Amination of Secondary over Primary C(sp3)-H Bonds.Molecules. 2022 Sep 21;27(19):6174. doi: 10.3390/molecules27196174. Molecules. 2022. PMID: 36234711 Free PMC article.
-
Highly efficient synthesis and stereoselective migration reactions of chiral five-membered aza-spiroindolenines: scope and mechanistic understanding.Chem Sci. 2016 Jul 1;7(7):4453-4459. doi: 10.1039/c6sc00176a. Epub 2016 Mar 29. Chem Sci. 2016. PMID: 30155093 Free PMC article.
-
Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase.Nat Chem Biol. 2014 Mar;10(3):209-15. doi: 10.1038/nchembio.1438. Epub 2014 Jan 26. Nat Chem Biol. 2014. PMID: 24463698 Free PMC article.
-
Efficient synthesis of 3H-indoles enabled by the lead-mediated α-arylation of β-ketoesters or γ-lactams using aryl azides.Org Lett. 2014 Jun 6;16(11):2916-9. doi: 10.1021/ol5010615. Epub 2014 May 27. Org Lett. 2014. PMID: 24865180 Free PMC article.
-
Mononuclear complexes of a tridentate redox-active ligand with sulfonamido groups: structure, properties, and reactivity.Chem Sci. 2018 Jul 2;9(31):6540-6547. doi: 10.1039/c7sc05445a. eCollection 2018 Aug 21. Chem Sci. 2018. PMID: 30310585 Free PMC article.
References
-
-
For recent, leading reviews, see: Wasilke JC, Obrey SJ, Baker RT, Bazan GC. Chem Rev. 2005;105:1001.Nicolaou KC, Edmonds DJ, Bulger PG. Angew Chem, Int Ed. 2006;45:7134.Padwa A. J Org Chem. 2009;74:6421.Grant TN, Rieder CJ, West FG. Chem Commun. 2009:5676.Touré BB, Hall DG. Chem Rev. 2009;109:4439.Lu LQ, Chen JR, Xiao WJ. Acc Chem Res. 2012;45:1278.
-
-
-
For recent reports, cf. Green JC, Pettus TRR. J Am Chem Soc. 2010;133:1603.Zou YQ, Lu LQ, Fu L, Chang NJ, Rong J, Chen JR, Xiao WJ. Angew Chem, Int Ed. 2011;50:7171.Tietze LF, Hungerland T, Düfert A, Objartel I, Stalke D. Chem Eur J. 2012;18:3286.Okazaki E, Okamoto R, Shibata Y, Noguchi K, Tanaka K. Angew Chem, Int Ed. 2012;51:6722.Jansone-Popova S, May JA. J Am Chem Soc. 2012;134:17877.
-
-
-
For recent reviews of C–H bond amination, see: Davies HML, Manning JR. Nature. 2008;451:417.Collet F, Dodd RH, Dauban P. Chem Commun. 2009:5061.Driver TG. Org Biomol Chem. 2010;8:3831.Du Bois J. Org Process Res Dev. 2011;15:758.Davies HML, Du Bois J, Yu JQ. Chem Soc Rev. 2011;40:1855.Roizen JL, Harvey ME, Du Bois J. Acc Chem Res. 2012;45:911.
-
-
-
For leading reports of N-atom transfer, see: Espino CG, Du Bois J. Angew Chem, Int Ed. 2001;40:598.Espino CG, Fiori KW, Kim M, Du Bois J. J Am Chem Soc. 2004;126:15378.Lebel H, Huard K, Lectard S. J Am Chem Soc. 2005;127:14198.Fraunhoffer KJ, White MC. J Am Chem Soc. 2007;129:7274.Liang C, Collet F, Robert-Peillard F, Müller P, Dodd RH, Dauban P. J Am Chem Soc. 2008;130:343.Reed SA, White MC. J Am Chem Soc. 2008;130:3316.Milczek E, Boudet N, Blakey S. Angew Chem, Int Ed. 2008;47:6825.Nguyen AI, Zarkesh RA, Lacy DC, Thorson MK, Heyduk AF. Chem Sci. 2011;2:166.
-
-
- Tsang WCP, Zheng N, Buchwald SL. J Am Chem Soc. 2005;127:14560. - PubMed
- Thu HY, Yu WY, Che CM. J Am Chem Soc. 2006;128:9048. - PubMed
- Mei TS, Wang X, Yu JQ. J Am Chem Soc. 2009;131:10806. - PubMed
- Tan Y, Hartwig JF. J Am Chem Soc. 2010;132:3676. - PubMed
- Kim JY, Park SH, Ryu J, Cho SH, Kim SH, Chang S. J Am Chem Soc. 2012;134:9110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources