Gold nanoparticle imaging and radiotherapy of brain tumors in mice
- PMID: 23265347
- PMCID: PMC3657324
- DOI: 10.2217/nnm.12.165
Gold nanoparticle imaging and radiotherapy of brain tumors in mice
Abstract
Aim: To test intravenously injected gold nanoparticles for x-ray imaging and radiotherapy enhancement of large, imminently lethal, intracerebral malignant gliomas.
Materials & methods: Gold nanoparticles approximately 11 nm in size were injected intravenously and brains imaged using microcomputed tomography. A total of 15 h after an intravenous dose of 4 g Au/kg was administered, brains were irradiated with 30 Gy 100 kVp x-rays.
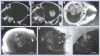
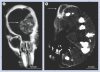
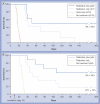
Results: Gold uptake gave a 19:1 tumor to normal brain ratio with 1.5% w/w gold in tumor, calculated to increase local radiation dose by approximately 300%. Mice receiving gold and radiation (30 Gy) demonstrated 50% long term (>1 year) tumor-free survival, whereas all mice receiving radiation only died.
Conclusion: Intravenously injected gold nanoparticles cross the blood-tumor barrier, but are largely blocked by the normal blood-brain barrier, enabling high-resolution computed tomography tumor imaging. Gold radiation enhancement significantly improved long-term survival compared with radiotherapy alone. This approach holds promise to improve therapy of human brain tumors and other cancers.
Figures


References
-
- Mrugala MM, Adair JE, Kiem HP. Outside the box: novel therapeutic strategies for glioblastoma. Cancer J. 2012;18(1):51–58. - PubMed
-
- Liu HL, Yang HW, Hua MY, Wei KC. Enhanced therapeutic agent delivery through magnetic resonance imaging-monitored focused ultrasound blood–brain barrier disruption for brain tumor treatment: an overview of the current preclinical status. Neurosurg. Focus. 2012;32(1):E4. - PubMed
-
- Parvez T. Present trend in the primary treatment of aggressive malignant glioma: glioblastoma multiforme. Technol. Cancer Res. Treat. 2008;7(3):241–248. - PubMed
-
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004;49(18):N309–N315. - PubMed
-
■ The first report of gold nanoparticle tumor radiotherapy enhancement in vivo.
Website
-
- NIH ImageJ software. ImageJ analysis download (program freely available) http://rsbweb.nih.gov/ij/download.html.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
