Intravenous artesunate versus intravenous quinine in the treatment of severe falciparum malaria: a retrospective evaluation from a UK centre
- PMID: 23265377
- PMCID: PMC4001579
- DOI: 10.1179/2047773212Y.0000000032
Intravenous artesunate versus intravenous quinine in the treatment of severe falciparum malaria: a retrospective evaluation from a UK centre
Abstract
Introduction: Despite evidence from developing world trials that intravenous (IV) artesunate (AS) is superior to IV quinine (Q) in severe falciparum malaria (FM), IV AS remains unlicensed in the UK with national guidelines listing it as an acceptable alternative to IV Q as the drug of choice. We retrospectively evaluate the safety and effectiveness of IV AS in returning travellers with severe FM.
Methods: We identified adults admitted to the Infectious Diseases unit with severe FM and treated with IV Q (1991-2009) or IV AS (2009-2011). Outcomes included adverse events, mortality, length of stay, admission to intensive care and, where data were available, parasite/fever clearance time and hypoglycaemic events.
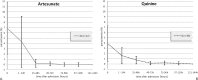
Results: Of 167 patients, 24 received IV AS and 143 IV Q. There was one potential AS-associated adverse event, a case of late onset haemolysis. Median length of stay (LOS) was significantly shorter for AS (3·5 versus 5 days, P = 0·017), even after adjusting for African ethnicity (for LOS ≥3 days, mhor = 0·33, P = 0·027; crude OR = 0·29, P = 0·013). In the AS group, there were no fatalities (versus five in Q group, NS) and fewer intensive care unit (ICU) admissions (NS). Median parasite clearance was significantly faster in AS (65 versus 85 hours in Q, P = 0·0045) with no hypoglycaemic episodes (versus five in Q).
Discussion: We found IV AS to be safe and effective, with shorter LOS, faster parasite and fever clearance, no fatalities or hypoglycaemic events, and fewer ICU admissions versus IV Q. This corroborates both developing world trials and smaller European case series (although these lacked comparison groups). As well as obvious benefits for patients, there are potential resource savings. A case of late-onset haemolysis may represent an adverse event, particularly as it has been documented elsewhere, warranting further investigation. Nonetheless, our experience suggests IV AS should be first-line for treating severe FM in the UK.
Figures
Similar articles
-
Intravenous artesunate plus oral dihydroartemisinin-piperaquine or intravenous quinine plus oral quinine for optimum treatment of severe malaria: lesson learnt from a field hospital in Timika, Papua, Indonesia.Malar J. 2019 Dec 30;18(1):448. doi: 10.1186/s12936-019-3085-3. Malar J. 2019. PMID: 31888655 Free PMC article.
-
Artesunate versus quinine in the treatment of severe imported malaria: comparative analysis of adverse events focussing on delayed haemolysis.Malar J. 2013 Jul 15;12:241. doi: 10.1186/1475-2875-12-241. Malar J. 2013. PMID: 23855745 Free PMC article.
-
Artemether for severe malaria.Cochrane Database Syst Rev. 2019 Jun 18;6(6):CD010678. doi: 10.1002/14651858.CD010678.pub3. Cochrane Database Syst Rev. 2019. PMID: 31210357 Free PMC article.
-
Post-treatment haemolysis in African children with hyperparasitaemic falciparum malaria; a randomized comparison of artesunate and quinine.BMC Infect Dis. 2017 Aug 17;17(1):575. doi: 10.1186/s12879-017-2678-0. BMC Infect Dis. 2017. PMID: 28818049 Free PMC article. Clinical Trial.
-
Artesunate, artemether or quinine in severe Plasmodium falciparum malaria?Expert Rev Anti Infect Ther. 2007 Apr;5(2):199-204. doi: 10.1586/14787210.5.2.199. Expert Rev Anti Infect Ther. 2007. PMID: 17402835 Review.
Cited by
-
UK malaria treatment guidelines 2016.J Infect. 2016 Jun;72(6):635-649. doi: 10.1016/j.jinf.2016.02.001. Epub 2016 Feb 12. J Infect. 2016. PMID: 26880088 Free PMC article.
-
The role of red blood cell exchange for severe imported malaria in the artesunate era: a retrospective cohort study in a referral centre.Malar J. 2016 Apr 14;15:216. doi: 10.1186/s12936-016-1264-z. Malar J. 2016. PMID: 27075988 Free PMC article.
-
Severe falciparum malaria treated with artesunate complicated by delayed onset haemolysis and acute kidney injury.Malar J. 2015 Jun 18;14:246. doi: 10.1186/s12936-015-0760-x. Malar J. 2015. PMID: 26081927 Free PMC article.
-
Development of Delayed Hemolytic Anemia After Treatment with Oral Artemether-Lumefantrine in Two Patients with Severe Falciparum Malaria.Am J Trop Med Hyg. 2017 May;96(5):1185-1189. doi: 10.4269/ajtmh.16-0460. Epub 2017 Feb 13. Am J Trop Med Hyg. 2017. PMID: 28193740 Free PMC article.
-
Systematic analysis of direct antiglobulin test results in post-artesunate delayed haemolysis.Malar J. 2021 Apr 29;20(1):206. doi: 10.1186/s12936-021-03735-w. Malar J. 2021. PMID: 33926462 Free PMC article.
References
-
- Health Protection Agency. Health Prot. Rep. 2011;5:17–28. Available from: http://www.hpa.org.uk/hpr/archives/2011/hpr1711.pdf(accessed 28/03/2012)
-
- Phillips A, Bassett P, Zeki S, Newman S, Pasvol G. Risk factors for severe disease in adults with falciparum malaria. Clin Infect Dis. 2009;48(7):871–8. - PubMed
-
- Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. - PubMed
-
- Lalloo DG, Shingadia D, Pasvol G, Chiodini PL, Whitty CJ, Beeching NJ, et al. UK malaria treatment guidelines. J Infect. 2007;54(2):111–21. - PubMed
-
- British Infection Society. Malaria — Algorithm for Initial Assessment and Management in Adults. Princes Risborough: BIS; 2007. Available from: http://www.britishinfection.org/drupal/sites/default/files/MalariaAlgori... (accessed 28/03/2012)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous