Diacylglycerol kinase θ: regulation and stability
- PMID: 23266086
- PMCID: PMC3992713
- DOI: 10.1016/j.jbior.2012.09.007
Diacylglycerol kinase θ: regulation and stability
Abstract
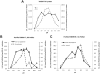
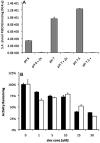
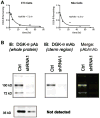
Given the well-established roles of diacylglycerol (DAG) and phosphatidic acid (PtdOH) in a variety of signaling cascades, it is not surprising that there is an increasing interest in understanding their physiological roles and mechanisms that regulate their cellular levels. One class of enzymes capable of coordinately regulating the levels of these two lipids is the diacylglycerol kinases (DGKs). These enzymes catalyze the transfer of the γ-phosphate of ATP to the hydroxyl group of DAG, which generates PtdOH while reducing DAG. As these enzymes reciprocally modulate the relative levels of these two signaling lipids, it is essential to understand the regulation and roles of these enzymes in various tissues. One system where these enzymes play important roles is the nervous system. Of the ten mammalian DGKs, eight of them are readily detected in the mammalian central nervous system (CNS): DGK-α, DGK-β, DGK-γ, DGK-η, DGK-ζ, DGK-ι, DGK-ε, and DGK-θ. Despite the increasing interest in DGKs, little is known about their regulation. We have focused some attention on understanding the enzymology and regulation of one of these DGK isoforms, DGK-θ. We recently showed that DGK-θ is regulated by an accessory protein containing polybasic regions. We now report that this accessory protein is required for the previously reported broadening of the pH profile observed in cell lysates in response to phosphatidylserine (PtdSer). Our data further reveal DGK-θ is regulated by magnesium and zinc, and sensitive to the known DGK inhibitor R599022. These data outline new parameters involved in regulating DGK-θ.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
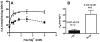

References
-
- Almena M, Merida I. Shaping up the membrane: diacylglycerol coordinates spatial orientation of signaling. Trends Biochem Sci. 2011;36(11):593–603. - PubMed
-
- Baldanzi G, Alchera E, Imarisio C, Gaggianesi M, Dal Ponte C, Nitti M, et al. Negative regulation of diacylglycerol kinase theta mediates adenosine-dependent hepatocyte preconditioning. Cell Death iffer. 2010;17(6):1059–68. - PubMed
-
- Biron D, Shibuya M, Gabel C, Wasserman SM, Clark DA, Brown A, et al. A diacylglycerol kinase modulates long-term thermotactic behavioral plasticity in C. elegans. Nat Neurosci. 2006;9(12):1499–505. - PubMed
-
- Brose N, Betz A, Wegmeyer H. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr Opin Neurobiol. 2004;14(3):328–40. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

